Ultimo aggiornamento 2022-05-27 14:11:19
Pazienti “poor responders” sono donne che hanno una scarsa e lenta risposta alla stimolazione ovarica  con produzione di ovociti in scarso numero e di scadente qualità, ridotto picco di estradiolo, basse percentuali di fertilizzazione e annidamento, ridotto outcome gravidico. In genere si tratta di donne in età avanzata (⩾40 anni), conta dei follicoli antrali (AFC) <7 o ormone anti-Mülleriano (AMH) <1,1 ng/ml].
con produzione di ovociti in scarso numero e di scadente qualità, ridotto picco di estradiolo, basse percentuali di fertilizzazione e annidamento, ridotto outcome gravidico. In genere si tratta di donne in età avanzata (⩾40 anni), conta dei follicoli antrali (AFC) <7 o ormone anti-Mülleriano (AMH) <1,1 ng/ml].
Nella nostra esperienza rappresentano il 10% circa delle pazienti FIV.
EZIOLOGIA: senescenza ovarica legata all’età delle pazienti, sempre più frequentemente over 40, oppure secondaria a fattori patologici come flogosi ed infezioni pelviche, traumi, interventi chirurgici, terapia radiante, chemioterapia, polimorfismo dell’aromatasi (3’UTR 1672 C→T), enzima che consente la conversione degli androgeni in estrogeni (1-6).
SINTOMATOLOGIA e DIAGNOSTICA – Le pazienti “Poor Responders” sono caratterizzate dalla presenza di uno o più fattori sfavorevoli:
1° – Elevata concentrazione sierica di FSH al 3° giorno di un ciclo spontaneo – la concentrazione sierica normale si pone a 9-10 mUI/ml; già valori di 12-13 mUI/ml sono predittivi di una scarsa risposta alla stimolazione ovarica controllata (COH) in termini di scarso numero di ovociti ma di buona qualità (36). Concentrazioni di 13-15 mUI/ml al 3° giorno indicano una grave insufficienza ovarica con scarsissima risposta alla COH, ma in entrambi questi ultimi due gruppi è possibile ottenere una gravidanza aumentando le dosi di r-FSH (450-600 UI/die) o aggiungendo LH esogeno (LH-added o HMG). Concentrazioni di FSH >17 mUI/ml livelli escludono qualsiasi possibilità di stimolazione ovarica (“no go” level); per queste pazienti l’unica possibilità di gravidanza è data dall’ovodonazione. Valori sierici basali di FSH <9 classicamente definiscono una paziente high responder.
2° – Estradiolo basale >85 pg/ml: è un test relativamente nuovo. Si effettua al 3° giorno del ciclo. Di per sè una concentrazione sierica così elevata di estradiolo non è un problema in se stesso ma essa può mascherare un elevato FSH basale che sarebbe evidenziabile se l’E2 fosse normale.
3° – Picco ridotto di E2: Acosta per primo definì poor responders le pazienti con picco di E2 <300 pg/ml dopo 7 giorni di stimolazione con HMG 150 UI/die.
4° – BMI (Body Mass Index) è il risultato del peso espresso in chilogrammi diviso per l’altezza in metri elevata al quadrato: Kg/m2. Le pazienti poor responders oversize (BMI >30) devono assolutamente rientrare in un peso accettabile (BMI <30) con dieta ed esercizi fisici prima di iniziare la stimolazione ovarica.
5° – Clomifene Challenge Test (CCCT): utilizzato per valutare la riserva ovarica specialmente in pazienti >38 anni di età, con precedenti fallimenti di cicli PMA, con infertilità inspiegata, con segni indiretti di ridotta 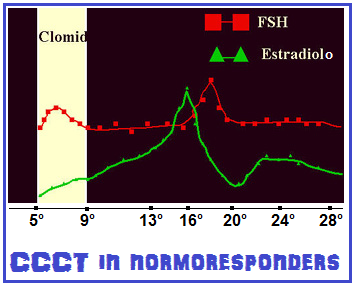 riserva ovarica come polimenorrea e quelle con livelli sierici di FSH >10 mUI/ml al 3° giorno del ciclo o con livelli di E2 >80 pg/ml. Al 3° giorno del ciclo si effettua il dosaggio sierico di E2 e FSH. Si somministra Clomid 100 mg/die dal 5° al 9° giorno del ciclo. Al 10-11° giorno del ciclo si ripete il dosaggio di E2 e FSH. In pazienti normoresponders i livelli di FSH inizialmente si innalzano e stimolano la crescita follicolare ma subito dopo (10-11° giorno) si abbassano correlativamente ed inversamente all’aumento di estradiolo (feed-back-negativo) fino a rientrare in valori normali (4-5 mUI/ml). Un livello sierico di FSH con modesto aumento (12-13 mUI/ml) sarà predittivo di una scarsa risposta in termini di numero di ovociti ma di buona qualità (36); valori sierici di FSH di 13-15 UI/ml sono correlati ad ancora più scarsa risposta alla stimolazione ovarica ma in entrambi questi gruppi sono possibili gravidanze aumentando i dosaggi delle gonadotropine o ricorrendo alla somministrazione addizionale di LH (LH-added); livelli di FSH >15 mUI/ml come valori base o al 10-11° giorno di CCCT fanno escludere qualsiasi tentativo di ottenere una gravidanza se non ricorrendo a ovodonazione. Il CCCT ha un basso indice di falsi positivi (<1%) ma un alto indice di falsi negativi (40% circa), cioè il riscontro di valori di FSH anomali indica sicuramente una ridotta riserva ovarica ma, viceversa, un CCCT negativo con valori normali di FSH non ci rassicura sulle possibilità di una soddisfacente risposta alla stimolazione ovarica.
riserva ovarica come polimenorrea e quelle con livelli sierici di FSH >10 mUI/ml al 3° giorno del ciclo o con livelli di E2 >80 pg/ml. Al 3° giorno del ciclo si effettua il dosaggio sierico di E2 e FSH. Si somministra Clomid 100 mg/die dal 5° al 9° giorno del ciclo. Al 10-11° giorno del ciclo si ripete il dosaggio di E2 e FSH. In pazienti normoresponders i livelli di FSH inizialmente si innalzano e stimolano la crescita follicolare ma subito dopo (10-11° giorno) si abbassano correlativamente ed inversamente all’aumento di estradiolo (feed-back-negativo) fino a rientrare in valori normali (4-5 mUI/ml). Un livello sierico di FSH con modesto aumento (12-13 mUI/ml) sarà predittivo di una scarsa risposta in termini di numero di ovociti ma di buona qualità (36); valori sierici di FSH di 13-15 UI/ml sono correlati ad ancora più scarsa risposta alla stimolazione ovarica ma in entrambi questi gruppi sono possibili gravidanze aumentando i dosaggi delle gonadotropine o ricorrendo alla somministrazione addizionale di LH (LH-added); livelli di FSH >15 mUI/ml come valori base o al 10-11° giorno di CCCT fanno escludere qualsiasi tentativo di ottenere una gravidanza se non ricorrendo a ovodonazione. Il CCCT ha un basso indice di falsi positivi (<1%) ma un alto indice di falsi negativi (40% circa), cioè il riscontro di valori di FSH anomali indica sicuramente una ridotta riserva ovarica ma, viceversa, un CCCT negativo con valori normali di FSH non ci rassicura sulle possibilità di una soddisfacente risposta alla stimolazione ovarica.
6° – Numero ridotto di piccoli (2-6 mm) follicoli antrali: la riserva ovarica viene valutata con scansione ecografica al 3° giorno del ciclo. Un ovaio con normale riserva follicolare evidenzia >10 piccoli follicoli antrali mentre valori <5 follicoli/ovaio sono propri delle pazienti poor responders.
7° – AMH (Anti-Mullerian Hormone) attualmente rappresenta il marker più affidabile per la valutazione della riserva ovarica; valori sierici <1.2 ng/ml sono indicativi di scarsa riserva ovarica (29-33).
8° – Ridotta concentrazione sierica di β-inibina: valori sierici <1,5 pg/ml indicano una scarsa riserva ovarica mentre valori >4,5 pg/ml sono predittivi di otcome gravidico del 95%.
9° – EFORT test (Exogenous Follicle stimulating hormone Ovarian Reserve Test): dosaggio beta-inibina e E2 al 3° giorno del ciclo; somministrazione di FSH 300 UI; nuovo dosaggio di ß-inibina ed E2 dopo 24 ore. L’aumento di entrambi questi ormoni è correlato con una buona riserva ovarica (34,35).
PROTOCOLLI COH PER PAZIENTI “POOR RESPONDERS”
1°) Incremento delle dosi di gonadotropine: è il primo e più semplice dei suggerimenti a cui ricorrere. Ma il limite massimo utile non può superare le 450 UI di gonadotropine al giorno (r-FSH 300 UI + HMG 150 UI). Oltre questo limite i vantaggi sono estremamente ridotti o nulli; altresì è inutile somministrare gonadotropine esogene se è già presente un’ipergonadotropinemia (10).
2°) Short protocol: è il protocollo maggiormente utilizzato nelle poor responders. Prevede la somministrazione concomitante di gonadotropine ed analoghi-die per 3-5 giorni oppure Gn-RH-a depot (Decapeptyl 375 mg fl) al 2° giorno del ciclo. In tal modo si potenzia la fase iniziale di stimolazione attraverso la dismissione massiva della quota endogena di FSH ed LH (effetto flare-up). In caso di inadeguata risposta alla stimolazione ovarica occorre valutare il livello sierico di LH e in caso di valori <1 mUI/ml sarà molto utile la somministrazione di LH (LH added) dal 7° giorno (Luveris fl 75 UI/die).
3° Gn-RH-a long Protocol: prevede la somministrazione di Gn-RH-analogo depot al 21° giorno del ciclo precedente la stimolazione. Più recentemente si preferisce utilizzare analoghi low dose a somministrazione giornaliera (Decapeptyl® 0.1 mg una fiala s.c. dal 21° giorno e 0.05 mg dal 1° giorno del ciclo fino alla somministrazione di HCG). La somministrazione di gonadotropine inizia dal 2° giorno del ciclo fino a che il follicolo leader raggiunga18 mm di diametro medio. La dose di GN è 300 UI r-FSH/die e viene variata dal 6° giorno del ciclo in relazione alla risposta ovarica Se al 9° giorno del ciclo l’LH è <1 mUI/ml si sostituisce r-FSH con HMG oppure continuare con r-FSH e aggiunger LH (LH added) (Luveris fl 75 UI/die).
In presenza di follicoli ∅ 16-18 mm viene somministrato HCG 10.000 UI.
L’obiettivo del long-protocol è bloccare il surge dell’FSH nella fase premestruale e conseguentemente bloccare la discrepanza nello sviluppo dei follicoli (9-12).

Altri protocolli possono essere utilizzati in alternativa ai protocolli 2 e 3 in caso di fallimento di questi ultimi.
4°) CC + HMG + Antagonist delayed Protocol: prevede l’associazione sequenziale di Clomifene (150 mg/die dal 1° al 5° giorno) e HMG (450 UI/die dal 3° giorno) e antagonista (Cetrotide o Orgalutran fl 0.25 mg/die s,c.) dal 7-8° giorno o dal momento che il follicolo leader raggiunge i 14 mm di diametro medio. HCG 10.000 UI il giorno che il follicolo leader raggiunge i 18 mm di diametro. Pick-up 33-36 ore dopo l’iniezione di HCG. Supplementazione luteale con progesterone vaginale 200 mg/die e HCG 2.000 UI/die fino a diagnosi ecografica di gravidanza (13-16).
Il protocollo che prevede l’utilizzo degli antagonisti necessita di un maggior quantità di gonadotropine ma ottiene migliori risultati per quanto riguarda il numero di follicoli maturi, percentuale di fecondazione degli ovociti e pregnancy rate nelle donne >40 anni di età (v. tabella sottostante).
5°) Luteal Estradiol Protocol: si somministra estradiolo valeriato (Progynova cpr 2 mg): 2 mg x 2 volte al dì dal 21° giorno del ciclo precedente fino al 3° giorno di stimolazione ovarica. rFSH dal 2° giorno del ciclo, microdose Gn-RH-a dal 3° giorno del ciclo, HCG 10 UI dal 3° giorno del ciclo. Il razionale della somministrazione dell’estradiolo è quello di abbassare la concentrazione sierica di FSH nella fase pre-mestruale del ciclo precedente a quello della stimolazione come per il long protocol Gn-RH-a evitando gli effetti negativi del Gn-RH-a depot (17,18).
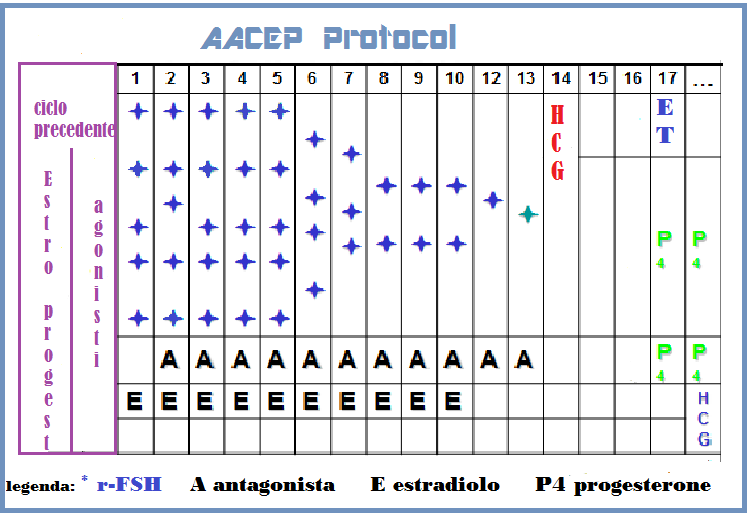
6°) AACEP Protocol (Gonadotropin-releasing hormone agonist/antagonist conversion with Estrogen Priming): particolarmente indicato in pazienti endometriosiche di età <42 anni che presentano all’anamnesi ripetuti fallimenti di cicli PMA anche utilizzando agonisti o agonisti. Questo protocollo intende ridurre la frequenza dell’apoptosi riferibile all’effetto flare-up LH dell’agonista e all’iperandrogenizzazione ovarica prodotta dagli antagonisti (19).
Si somministra la pillola estro-progestinica per 21 giorni, Gn-RH-a low dose negli ultimi 7 giorni della pillola e fino alla comparsa del ciclo; Estradiolo valeriato dal 1° al 10° giorno del ciclo, Gn-Rh antagonista low dose (0,125 ng/die) dal 2° giorno del ciclo, Estradiolo vaginale (Vagifen cpr vaginali 0,25 mg: 1 cp/die) dal 5° al 10° giorno, r-FSH 600 UI al giorno per i primi dal 2° al 4° giorno del ciclo e quindi a scalare fino a 225 UI/die (21).
7°) LH added: è da considerare in casi di iniziale inadegata risposta alla stimolazione ovarica e/o con severa soppressione di LH (valori sierici <1 mUI/ml) che può verificarsi utilizzando protocolli di CFM con analoghi o antagonisti del Gn-RH e nelle pazienti >35 anni di età (28). Non tutti gli AA. concordano sull’utilità dell’LH-added anche in queste estreme condizioni.
8°) Luteal-phase ovarian stimulation: stimolazione ovarica con letrozolo e HMG in fase luteale dopo ovulazione spontanea. La stimolazione è iniziata il giorno dopo un’ovulazione spontanea utilizzando letrozolo (2.5 mg/die) fino a che si il follicolo leader raggiunge un diametro >12 mm; anche la somministrazione di HMG alla dose di 225 UI/die inizia il giorno dopo l’ovulazione, in contemporanea con il letrozolo, ma viene prolungata fino a che almeno 3 follicoli raggiungono il diametro medio di 18 mm. In quel giorno la maturazione follicolare è sviluppata da 1/2 fiala (1,75 mg) di analogo depot i.m. ed il pick-up è effettuato 36 ore dopo (37,38). Il razionale della stimolazione ovarica in fase luteale si basa sull’esperienza consolidata che è possibile ottenere buoni ovociti anche in fase luteale continuando una stimolazione ovarica iniziata normalmente nella fase follicolare dello stesso ciclo (flexible ovarian stimulation) (39). Il razionale della somministrazione di letrozolo si basa sull’ipotesi che gli inibitori dell’aromatasi possano mimare l’azione del Clomifene nel ridurre il feedback negativo degli estrogeni sulla secrezione dell’ormone follicolo-stimolante (FSH), senza produrre l’intensa deplezione dei recettori estrogenici tipica del clomifene. Si viene a mimare un microambiente endocrino molto simile alle donne PCOS che notoriamente sono iperresponsive alla stimolazione ovarica. I risultati dei primi studi pubblicati sembrano lusinghieri presentando un PR del 50% circa (37).
Supplementazione luteale: la concentrazione plasmatica di progesterone ed estradiolo nei cicli IVF tende a diminuire bruscamente in fase luteale. Si è cercato di porre rimedio a tale situazione con la somministrazione di progesterone micronizzato ed estradiolo valerato. Molteplici trial clinici randomizzati effettuati da diversi AA. concordano nel dimostrare la validità della supplementazione progestinica e/o HCG in termini di significativo aumento dell’outcome gravidico. Viceversa nessun miglioramento si osserva nei cicli supplementati con estradiolo (23-27). Interessante ma da verificare è lo studio di AA. messicani sulla somministrazione vaginale di Sildanefil in poor responder che presentano scarso aumento del thickness endometriale (<7 mm) al giorno del pich-up di un prcedente ciclo IVF. Il Sildanefil avrebbe un effetto eutrofizzante sull’endometrio e favorirebbe l’annidamento (28).
- progesterone per applicazione vaginale (Progeffik, Prometrium gel vaginale o cpr 200 mg/die; Crinone 8 gel vaginale: 1-2 applicazioni al dì). La somministrazione di P per via intramuscolare (Prontogest fl 100 mg: 1/2 fl al dal giorno del transfer per 21 giorni) presenta gli stessi risultati ma ovviamente è meno gradita alle pazienti. La somministrazione di didrogesterone orale è ancor più gradita dalle pazienti ma presenta un minor outcome gravidico.
- HCG 2.000 UI/die: è più efficace, incrementa la secrezione di progesterone ed estradiolo ma presenta il rischio di iperstimolazione ovarica severa (OHSS) (15).
- E2: 2-6 mg/die di estradiolo valeriato (Progynova cpr 2 mg) dal giorno dell’embryo transfer per 21 giorni.
- Sildanefil: una compressa da 50 mg/die è inserita in vagina dal 9° giorno di stimolazione e fino al giorno pecedente l’embryo transfer. L’unico effetto collaterale osservato del farmaco era un lieve arrossamento vaginale che scompariva spontaneamente dopo la sospensione del farmaco.
- Antiossidanti: la somministrazione di fattori antiossidanti come la Vitamina C, vitamina E e carotenoidi sembra influenzare positivamente l’oucome ovocitario e gravidico come dimostrato dal rilevamento di alte concentrazioni sieriche di tali fattori in donne sottoposte a cicli FIV e risultate gravide rispetto al gruppo di pazienti in cui la FIV non ha dato esito positivo (40).
Bibliografia:
- Behre HM, Greb RR, Mempel A, Sonntag B, Kiesel L, Kaltwaßer P, Selinger E, Röpke F, Gromoll J and Simoni M (2005) Significance of a common single nucleotide polymorphism in exon 10 of the follicle-stimulating hormone (FSH) receptor gene for ovarian response to FSH: a pharmacogenetic approach to controlled ovarian hyperstimulation. Pharmcogen Genom 15,451”“456.
- Daelemans C, Smits G, de Maertelaer V, Costagliola S, Englert Y, Vassart G and Delbaere A (2004) Prediction of severity of symptoms in iatrogenic ovarian hyperstimulation syndrome by follicle-stimulating hormone receptor Ser680 Asn polymorphism. J Clin Endocrinol Metab 89,6310”“6315.
- De Castro F, Ruiz R, Montoro L, Pérez-Hernández D, Sánchez-Casas Padilla E, Real LM and Ruiz A (2003) Role of follicle-stimulating hormone receptor Ser680Asn polymorphism in the efficacy of follicle-stimulating hormone. Fertil Steril 80,571”“576.
- de Castro F, Moron FJ, Montoro L et al. 2004 Human controlled ovarian hyperstimulation outcome is a polygenic trait. Pharmacogenetics 14, 285”“293.
- De Castro F, Moron FJ, Montoro L, Galan JJ, Real LM, Ruiz A Re: polymorphisms associated with circulating sex hormone levels in postmenopausal women. J. Natl. Cancer Inst. 97, 152”“153 (2005).
- de Koning C.H., T.Benjamins, P.Harms, R.Homburg, J.M.van Montfrans, J.Gromoll, M.Simoni and C.B.Lambalk The distribution of FSH receptor isoforms is related to basal FSH levels in subfertile women with normal menstrual cycles Human Reproduction Vol.21, No.2 pp. 443”“446, 2006
- Battaglia DE, Goodwin P, Klein NA, Soules MR: “Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women”. Hum Reprod 1996;11.2217-22.
- Angell R: “First meiotic-division nondisjunction in human oocytes”. Human Genet 1997;61:23-32.
- Pellestor F, Andreo B, Arnal F, Humeau C, Demaille J: “Maternal aging and chromosal abnormalities: new data drawn from in vitro unfertilized human oocytes”. Hum Genet 2003;112:195-203.
- Arslan M, Bocca S, Mirkin S, Barroso G, Stadtmauer L, Oehninger S. Controlled ovarian hyperstimulation protocols for in vitro fertilization: two decades of experience after the birth of Elizabeth Carr. Fertil Steril. 2005;84:555–569.
- Karande V, Gleicher N. A rational approach to the management of low responders in in-vitro fertilization. Hum Reprod. 1999;14:1744–1748.
- Garcia JE, Jones GS, Acosta AA, Wright G. Human menopausal gonadotropin/human chorionic gonadotropin follicular maturation for oocyte aspiration: phase II. Fertil Steril. 1981;39:174–179.
- Muasher SJ. Treatment of low responders. J Assist Reprod Genet. 1993;10:112–114.
- Karande V, Gleicher N. A rational approach to the management of low responders in in-vitro fertilization. Hum Reprod. 1999;14:1744–1748.
- Schoolcraft W, Schlenker T, Gee M, Stevens J, Wagley L. Improved controlled ovarian hyperstimulation in poor responder in vitro fertilization patients with a microdose follicle-stimulating hormone flare, growth hormone protocol. Fertil Steril. 1997;67:93–97.
- Dragisic KG Fertil Steril 2005;84:1023-1026
- Frattarelli J, et al: “A luteal estradiol protocol for expected poor-responders improves embryo number and quality” Fertil Steril 2008;89,5:1118-22
- Kolibianakis EM, Albano C, et al: “Exposure to hihg levels luteinizing hormone and estradiol in thr early phase of gonadotropin releasing hormone antagonist cycles is associated with a reduced chance of pregnancy”. Fertil Steril 2003; 79:873-880.
- Fisch JD, Keskintepe L and Sher G: “Gonadotropin-releasing hormone agonist/antagonist conversion with estrogen priming in low responders with prior in vitro fertilization failure”. Fertil Steril 2008;89,2:342-347
- Martinez F: “ Human Corionic Gonadotropin and intravaginal natural progesterone are equally effective for luteal phase support in IVF”. Gynecol Endocrinol 2000; 14:316-320
- Lukaszuk K: “Estradio supplementation during the luteal phase in poor responder patients undergoing in vitro fertilization: a randomized clinical trial”. J Assist Reprod Genet 2011;28(9):785-790.
- Engmann L, Di Luigi A, Schmidt D, Benadiva C, Maier D, Nulsen J.: “The effect of luteal phase vaginal estradiol supplementation on the success of in vitro fertilization treatment: a prospective randomized study”. Fertil Steril. 2008 Mar;89(3):554-61.
- Serna J, Cholquevilque JL, Cela V, Martínez-Salazar J, Requena A, Garcia-Velasco JA.: “Estradiol supplementation during the luteal phase of IVF-ICSI patients: a randomized, controlled trial”. Fertil Steril. 2008 Dec;90(6):2190-5.
- Moini A, Zadeh Modarress S, Amirchaghmaghi E, Mirghavam N, Khafri S, Reza Akhoond M, Salman Yazdi R.: “The effect of adding oral oestradiol to progesterone as luteal phase support in ART cycles – a randomized controlled study”. Arch Med Sci. 2011 Feb;7(1):112-6
- Canona J et al: “The use of vaginal sildanefil in patients with poor endometrial response: The Mexican IVF experience”. Fertility and Sterility Volume 82, Supplement 2, Pages S197–S198, September 2004
- Suheil J. Muasher, Rony T. Abdallah, Ziad R. Hubayter: “Optimal stimulation protocols for in vitro fertilization” Fertil Steril 2006; 86,2:267-273
- Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level of anti-Mullerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod 2006;21:2022-6.
- Min Hye Choi, Ji Hee Yoo, Hye Ok Kim, Sun Hwa Cha, Chan Woo Park, Kwang Moon Yang, In Ok Song, Mi Kyoung Koong, Inn Soo Kang. Serum anti-Müllerian hormone levels as a predictor of the ovarian response and IVF outcomes. Clin Exp Reprod Med 2011;38(3):153-158
- Blazar AS, Lambert-Messerlian G, Hackett R, et al. Use of in-cycle antimüllerian hormone levels to predict cycle outcome. Am J Obstet Gynecol, 2011;205:223.
- Gnoth C, Shuring AN, Frid K, Tigger J. Relevance of AMH measurement in a routine IVF program. Hum Reprod, 2008;23:1359–65.
- Anti-Mullerian hormone measurement on any day of the menstrual cycle strongly predicts ovarian response in assisted reproductive technology Hum Reprod (2007) 22 (3): 766-771
- R. Fanchin, D. de Ziegler, F. Olivennes, J Taieb, A Dzik and R Frydman: “Endocrinology: Exogenous follicle stimulating hormone ovarian reserve test (EFORT): a simple and reliable screening test for detecting ‘poor responders’ in in-vitro fertilization”. Human Reprod 1994:9;9:1607-1611.
- Kwee J, Schats R, McDonnell J, Schoemaker J, Lambalk CB.: “The clomiphene citrate challenge test versus the exogenous folliclestimulating hormone ovarian reserve test as a single test for identification of low responders and hyperresponders to in vitro fertilization”. Fertil Steril. 2006;85:1714-22.
- M. Luna-Rojas, L. Grunfeld, B. Sandler, M. Duke, A.B. Copperman, J. Barritt: “Moderately elevated day-3 FSH levels predict egg quantity better than egg quality”. Fertility and Sterility September 2004 (Vol. 82, Page S127)
- Yanping Kuang, Qingqing Hong, Qiuju Chen,Qifeng Lyu, Ai Ai, Yonglun Fu, Zeev Shoham: “Luteal-phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen-thawed embryo transfer cycles”. Fertil Steril 2014;101,1:105-111.
- Bedoschi GM, Oliveira de Albuquerque F, Ferriani RA, Navarro PA: ” Ovarian stimulation during the luteal phase for fertility preservation of cancer patients: case reports and review of the literature”.
Journal of Assisted Reproduction and Genetics; 2010; 27,8:491-494
- Bin Xu, Yanping LI: “Flexible ovarian stimulation in a poor responder: a case report and literature review”. Reproductive BioMedicine Online; 2013; 26,4:378-383.
- Palini S. et al: Influence of ovarian stimulation for IVF/ICSI on the antioxidant defence system and relationship to outcome”. Reproductive BioMedicine Online; 2014;29,1:65-71.
- . Ubaldi FM, Rienzi L, Ferrero S, et al. Management of poor responders in IVF. Reprod Biomed Online 2005; 10: 235–246.
- Vaiarelli A, Cimadomo D, Ubaldi N, et al. What is new in the management of poor ovarian response in IVF. Curr Opin Obstet Gynecol 2018; 30: 155–162.
- Blumenfeld Z. What is the best regimen for ovarian stimulation of poor responders in ART/IVF. Front Endocrinol (Lausanne) 2020; 11: 192.
- Blumenfeld Z. Premature ovarian failure: etiology and possible prevention. Expert Rev Endocrinol Metab 2009; 4: 173–181.
- Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update 2005; 11: 391–410.
- Lee HC, Lyndon A, Blumenfeld YJ, et al. Antenatal steroid administration for premature neonates in California. Obstet Gynecol 2011; 117: 603–609.
- Skillern A, Rajkovic A. Recent developments in identifying genetic determinants of premature ovarian failure. Sex Dev 2008; 2: 228–243.
- Drakopoulos P, Vuong TNL, Ho NAV, et al. Corifollitropin alfa followed by highly purified HMG versus recombinant FSH in young poor ovarian responders: a multicentre randomized controlled clinical trial. Hum Reprod 2017; 32: 2225–2233.
- Polyzos NP, Devroey P. A systematic review of randomized trials for the treatment of poor ovarian responders: is there any light at the end of the tunnel. Fertil Steril 2011; 96: 1058–1061.
- Ferraretti AP, La Marca A, Fauser BC, et al. ESHRE consensus on the definition of “poor response” to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod 2011; 26: 1616–1624.
- La Marca A, Grisendi V, Giulini S, et al. Live birth rates in the different combinations of the Bologna criteria poor ovarian responders: a validation study. J Assist Reprod Genet 2015; 32: 931–937.
- Polyzos NP, Nwoye M, Corona R, et al. Live birth rates in Bologna poor responders treated with ovarian stimulation for IVF/ICSI. Reprod Biomed Online 2014; 28: 469–474.
- Busnelli A, Papaleo E, Del Prato D, et al. A retrospective evaluation of prognosis and cost-effectiveness of IVF in poor responders according to the Bologna criteria. Hum Reprod 2015; 30: 315–322.
- Boza A, Oguz SY, Misirlioglu S, et al. Utilization of the Bologna criteria: a promise unfulfilled? A review of published and unpublished/ongoing trials. Fertil Steril 2018; 109: 104–109.
- Frydman R. Poor responders: still a problem. Fertil Steril 2011; 96: 1057.
- Papathanasiou A. Implementing the ESHRE “poor responder” criteria in research studies: methodological implications. Hum Reprod 2014; 29: 1835–1838. [PubMed] [Google Scholar]
- Younis JS. The Bologna criteria for poor ovarian response; has the job been accomplished? Hum Reprod 2012; 27: 1874–1875; author reply 1875–1876. [PubMed] [Google Scholar]
- Bozdag G, Polat M, Yarali I, et al. Live birth rates in various subgroups of poor ovarian responders fulfilling the Bologna criteria. Reprod Biomed Online 2017; 34: 639–644.
- Romito A, Bardhi E, Errazuriz J, et al. Heterogeneity among poor ovarian responders according to Bologna criteria results in diverging cumulative live birth rates. Front Endocrinol (Lausanne) 2020; 11: 208.
- Xu B, Chen Y, Geerts D, et al. Cumulative live birth rates in more than 3,000 patients with poor ovarian response: a 15-year survey of final in vitro fertilization outcome. Fertil Steril 2018; 109: 1051–1059.
- Polyzos NP, Corona R, Van De Vijver A, et al. Corifollitropin alfa followed by hpHMG in GnRH agonist protocols. Two prospective feasibility studies in poor ovarian responders. Gynecol Endocrinol 2015; 31: 885–890.
- Polyzos NP, Drakopoulos P, Tournaye H. Modified natural cycle IVF for poor ovarian responders: rethink before concluding. Hum Reprod 2016; 31: 221–222. [PubMed] [Google Scholar]
- Errázuriz J, Romito A, Drakopoulos P, et al. Cumulative live birth rates following stimulation with corifollitropin alfa compared with hp-hMG in a GnRH antagonist protocol in poor ovarian responders. Front Endocrinol (Lausanne) 2019; 10: 175.
- Errázuriz J, Drakopoulos P, Pening D, et al. Pituitary suppression protocol among Bologna poor responders undergoing ovarian stimulation using corifollitropin alfa: does it play any role. Reprod Biomed Online 2019; 38: 1010–1017.
- Poseidon Group (Patient-Oriented Strategies Encompassing IndividualizeD Oocyte Number), Alviggi C, Andersen CY, et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril 2016; 105: 1452–1453.
- Humaidan P, Alviggi C, Fischer R, et al. The novel POSEIDON stratification of “Low prognosis patients in Assisted Reproductive Technology” and its proposed marker of successful outcome. F1000Res 2016; 5: 2911. [PMC free article] [PubMed] [Google Scholar]
- Esteves SC, Carvalho JF, Bento FC, et al. A novel predictive model to estimate the number of mature oocytes required for obtaining at least one euploid blastocyst for transfer in couples undergoing in vitro fertilization/intracytoplasmic sperm injection: the ART calculator. Front Endocrinol (Lausanne) 2019; 10: 99. [PMC free article] [PubMed] [Google Scholar]
- Esteves SC, Yarali H, Ubaldi FM, et al. Validation of ART calculator for predicting the number of metaphase II oocytes required for obtaining at least one euploid blastocyst for transfer in couples undergoing in vitro fertilization/intracytoplasmic sperm injection. Front Endocrinol (Lausanne) 2019; 10: 917.
- Haahr T, Dosouto C, Alviggi C, et al. Management strategies for POSEIDON groups 3 and 4. Front Endocrinol (Lausanne) 2019; 10: 614.
- Alsbjerg B, Haahr T, Elbaek HO, et al. Dual stimulation using corifollitropin alfa in 54 Bologna criteria poor ovarian responders—a case series. Reprod Biomed Online 2019; 38: 677–682.
- Pu D, Wu J, Liu J. Comparisond meta-analysis accounting for patient type. Hum Reprod Update 2017; 23: 560–579.
- Sunkara SK, Coomarasamy A, Faris R, et al. Long gonadotropin-releasing hormone agonist versus short agonist versus antagonist regimens in poor responders undergoing in vitro fertilization: a rands of GnRH antagonist versus GnRH agonist protocol in poor ovarian responders undergoing IVF. Hum Reprod 2011; 26: 2742–2749.
- Lambalk CpracticeB, Banga FR, Huirne JA, et al. GnRH antagonist versus long agonist protocols in IVF: a systematic review an randomized controlled trial. Fertil Steril 2014; 101: 147–153.
- Humaidan P, La Marca A, Alviggi C, et al. Future perspectives of POSEIDON stratification for clinical and research. Front Endocrinol (Lausanne) 2019; 10: 439.
- Blockeel C, Riva A, De Vos M, et al. Administration of a gonadotropin-releasing hormone antagonist during the 3 days before the initiation of the in vitro fertilization/intracytoplasmic sperm injection treatment cycle: impact on ovarian stimulation. Fertil Steril 2011; 95: 1714–1719.
- Fischer R, Nakano FY, Roque M, et al. A quality management approach to controlled ovarian stimulation in assisted reproductive technology: the “Fischer protocol.” Panminerva Med 2019; 61: 11–23.
- Ubaldi F, Vaiarelli A, D’Anna R, et al. Management of poor responders in IVF: is there anything new. Biomed Res Int 2014; 2014: 352098.
- Group EREG. Guideline on ovarian stimulation for IVF/ICSI. Grimbergen: European Society of Human Reproduction and Embryology, 2019.
- Berkkanoglu M, Ozgur K. What is the optimum maximal gonadotropin dosage used in microdose flare-up cycles in poor responders. Fertil Steril 2010; 94: 662–665.
- Baker VL, Brown MB, Luke B, et al. Gonadotropin dose is negatively correlated with live birth rate: analysis of more than 650,000 assisted reproductive technology cycles. Fertil Steril 2015; 104: 1145–1152.e5.
- Bosch E, Labarta E, Crespo J, et al. Impact of luteinizing hormone administration on gonadotropin-releasing hormone antagonist cycles: an age-adjusted analysis. Fertil Steril 2011; 95: 1031–1036. [PubMed] [Google Scholar]
- Hill MJ, Levens ED, Levy G, et al. The use of recombinant luteinizing hormone in patients undergoing assisted reproductive techniques with advanced reproductive age: a systematic review and meta-analysis. Fertil Steril 2012; 97: 1108–1114.e1.
- Lehert P, Kolibianakis EM, Venetis CA, et al. Recombinant human follicle-stimulating hormone (r-hFSH) plus recombinant luteinizing hormone versus r-hFSH alone for ovarian stimulation during assisted reproductive technology: systematic review and meta-analysis. Reprod Biol Endocrinol 2014; 12: 17.
- Humaidan P, Chin W, Rogoff D, et al. Efficacy and safety of follitropin alfa/lutropin alfa in ART: a randomized controlled trial in poor ovarian responders. Hum Reprod 2017; 32: 544–555.
- Alviggi C, Conforti A, Esteves SC, et al. Recombinant luteinizing hormone supplementation in assisted reproductive technology: a systematic review. Fertil Steril 2018; 109: 644–664.
- Practice Committee of the American Society for Reproductive Medicine. Comparison of pregnancy rates for poor responders using IVF with mild ovarian stimulation versus conventional IVF: a guideline. Fertil Steril 2018; 109: 993–999.
- Zhang Y, Zhang C, Shu J, et al. Adjuvant treatment strategies in ovarian stimulation for poor responders undergoing IVF: a systematic review and network meta-analysis. Hum Reprod Update 2020; 26: 247–263.
- Drakopoulos P, Romito A, Errázuriz J, et al. Modified natural cycle IVF versus conventional stimulation in advanced-age Bologna poor responders. Reprod Biomed Online 2019; 39: 698–703.
- Polyzos NP, Blockeel C, Verpoest W, et al. Live birth rates following natural cycle IVF in women with poor ovarian response according to the Bologna criteria. Hum Reprod 2012; 27: 3481–3486. [PubMed] [Google Scholar]
- Vaiarelli A, Cimadomo D, Trabucco E, et al. Double stimulation in the same ovarian cycle (DuoStim) to maximize the number of oocytes retrieved from poor prognosis patients: a multicenter experience and SWOT analysis. Front Endocrinol (Lausanne) 2018; 9: 317. [PMC free article] [PubMed] [Google Scholar]
- Vaiarelli A, Cimadomo D, Conforti A, et al. Luteal phase after conventional stimulation in the same ovarian cycle might improve the management of poor responder patients fulfilling the Bologna criteria: a case series. Fertil Steril 2020; 113: 121–130.
- Yeung T, Chai J, Li R, et al. A double-blind randomised controlled trial on the effect of dehydroepiandrosterone on ovarian reserve markers, ovarian response and number of oocytes in anticipated normal ovarian responders. BJOG 2016; 123: 1097–1105.
- Zhang M, Niu W, Wang Y, et al. Dehydroepiandrosterone treatment in women with poor ovarian response undergoing IVF or ICSI: a systematic review and meta-analysis. J Assist Reprod Genet 2016; 33: 981–991.
- Nagels HE, Rishworth JR, Siristatidis CS, et al. Androgens (dehydroepiandrosterone or testosterone) for women undergoing assisted reproduction. Cochrane Database Syst Rev 2015: CD009749.
- Polyzos NP, Davis SR, Drakopoulos P, et al. Testosterone for poor ovarian responders: lessons from ovarian physiology. Reprod Sci 2018; 25: 980–982.
- Drakopoulos P, Pluchino N, Bischof P, et al. Effect of growth hormone on endometrial thickness and fertility outcome in the treatment of women with panhypopituitarism: a case report. J Reprod Med 2016; 61: 78–82.
- Mason HD, Martikainen H, Beard RW, et al. Direct gonadotrophic effect of growth hormone on oestradiol production by human granulosa cells in vitro. J Endocrinol 1990; 126: R1–R4.
- Bachelot A, Monget P, Imbert-Bolloré P, et al. Growth hormone is required for ovarian follicular growth. Endocrinology 2002; 143: 4104–4112.
- Norman RJ, Alvino H, Hull LM, et al. Human growth hormone for poor responders: a randomized placebo-controlled trial provides no evidence for improved live birth rate. Reprod Biomed Online 2019; 38: 908–915.
- Xu Y, Nisenblat V, Lu C, et al. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: a randomized controlled trial. Reprod Biol Endocrinol 2018; 16: 29.
- Sfakianoudis K, Simopoulou M, Nitsos N, et al. A case series on platelet-rich plasma revolutionary management of poor responder patients. Gynecol Obstet Invest 2019; 84: 99–106.
- Stojkovska S, Dimitrov G, Stamenkovska N, et al. Live birth rates in poor responders’ group after previous treatment with autologous platelet-rich plasma and low dose ovarian stimulation compared with poor responders used only low dose ovarian stimulation before in vitro fertilization. Open Access Maced J Med Sci 2019; 7: 3184–3188. [
- Kawamura K, Cheng Y, Suzuki N, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A 2013; 110: 17474–17479.
- Kawamura K, Ishizuka B, Hsueh AJW. Drug-free in-vitro activation of follicles for infertility treatment in poor ovarian response patients with decreased ovarian reserve. Reprod Biomed Online 2020; 40: 245–253.
- Bardhi E, Blockeel C, Cools W, et al. Is ovarian response associated with adverse perinatal outcomes in GnRH antagonist IVF/ICSI cycles? Reprod Biomed Online. Epub ahead of print 11 April 2020. DOI: 10.1016/j.rbmo.2020.03.010.
- Perez Mayorga M, Gromoll J, Behre HM, et al. Ovarian response to follicle-stimulating hormone (FSH) stimulation depends on the FSH receptor genotype. J Clin Endocrinol Metab 2000; 85: 3365–3369.
- Alviggi C, Humaidan P, Howles CM, et al. Biological versus chronological ovarian age: implications for assisted reproductive technology. Reprod Biol Endocrinol 2009; 7: 101.
- Alviggi C, Pettersson K, Longobardi S, et al. A common polymorphic allele of the LH beta-subunit gene is associated with higher exogenous FSH consumption during controlled ovarian stimulation for assisted reproductive technology. Reprod Biol Endocrinol 2013; 11: 51.
- Lindgren I, Bååth M, Uvebrant K, et al. Combined assessment of polymorphisms in the LHCGR and FSHR genes predict chance of pregnancy after in vitro fertilization. Hum Reprod 2016; 31: 672–683.
- Sunkara SK, Polyzos NP. OPTIMIST trial: optimistic evidence? Hum Reprod 2018; 33: 983–984. [PubMed] [Google Scholar]
- Romanski PA, Farland LV, Tsen LC, et al. Effect of class III and class IV obesity on oocyte retrieval complications and outcomes. Fertil Steril 2019; 111: 294–301.
- Gallot V, Berwanger da, Silva AL, Genro V, et al. Antral follicle responsiveness to follicle-stimulating hormone administration assessed by the Follicular Output RaTe (FORT) may predict in vitro fertilization-embryo transfer outcome. Hum Reprod 2012; 27: 1066–1072.
- Alviggi C, Conforti A, Esteves SC, et al. Understanding ovarian hypo-response to exogenous gonadotropin in ovarian stimulation and its new proposed marker—the follicle-to-oocyte (FOI) index. Front Endocrinol (Lausanne) 2018; 9: 589.
- Drakopoulos P, Blockeel C, Stoop D, et al. Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum Reprod 2016; 31: 370–376.
- Conforti A, Esteves SC, Picarelli S, et al. Novel approaches for diagnosis and management of low prognosis patients in assisted reproductive technology: the POSEIDON concept. Panminerva Med 2019; 61: 24–29.
- Mohiyiddeen L, Newman WG, McBurney H, et al. Follicle-stimulating hormone receptor gene polymorphisms are not associated with ovarian reserve markers. Fertil Steril 2012; 97: 677–681.
- Polyzos NP, Drakopoulos P, Parra J, et al. Cumulative live birth rates according to the number of oocytes retrieved after the first ovarian stimulation for in vitro fertilization/intracytoplasmic sperm injection: a multicenter multinational analysis including approximately 15,000 women. Fertil Steril 2018; 110: 661–670.e1.
- Ata B, Kaplan B, Danzer H, et al. Array CGH analysis shows that aneuploidy is not related to the number of embryos generated. Reprod Biomed Online 2012; 24: 614–620.
- Drakopoulos P, Errazuriz J, Santos-Ribeiro S, et al. Cumulative live birth rates in in-vitro fertilization. Minerva Ginecol 2019; 71: 207–210.
- Devroey P, Pellicer A, Nyboe Andersen A, et al. A randomized assessor-blind trial comparing highly purified hMG and recombinant FSH in a GnRH antagonist cycle with compulsory single-blastocyst transfer. Fertil Steril 2012; 97: 561–571.
- Santi D, Casarini L, Alviggi C, et al. Efficacy of follicle-stimulating hormone (FSH) alone, FSH + luteinizing hormone, human menopausal gonadotropin or FSH + human chorionic gonadotropin on assisted reproductive technology outcomes in the “personalized” medicine era: a meta-analysis. Front Endocrinol (Lausanne) 2017; 8: 114.
- Behre HM, Greb RR, Mempel A, et al. Significance of a common single nucleotide polymorphism in exon 10 of the follicle-stimulating hormone (FSH) receptor gene for the ovarian response to FSH: a pharmacogenetic approach to controlled ovarian hyperstimulation. Pharmacogenet Genomics 2005; 15: 451–456.
- Drakopoulos P, Santos-Ribeiro S, Bosch E, et al. The effect of dose adjustments in a subsequent cycle of women with suboptimal response following conventional ovarian stimulation. Front Endocrinol (Lausanne) 2018; 9: 361.
- Martin JR, Bromer JG, Sakkas D, et al. Live babies born per oocyte retrieved in a subpopulation of oocyte donors with repetitive reproductive success. Fertil Steril 2010; 94: 2064–2068.
- De Placido G, Alviggi C, Perino A, et al. Recombinant human LH supplementation versus recombinant human FSH (rFSH) step-up protocol during controlled ovarian stimulation in normogonadotrophic women with initial inadequate ovarian response to rFSH. A multicentre, prospective, randomized controlled trial. Hum Reprod 2005; 20: 390–396.
- Papaleo E, Vanni VS, Viganò P, et al. Recombinant LH administration in subsequent cycle after “unexpected” poor response to recombinant FSH monotherapy. Gynecol Endocrinol 2014; 30: 813–816.
- Conforti A, Esteves SC, Di Rella F, et al. The role of recombinant LH in women with hypo-response to controlled ovarian stimulation: a systematic review and meta-analysis. Reprod Biol Endocrinol 2019; 17: 18. [PMC free article] [PubMed] [Google Scholar]
- Conforti A, Esteves SC, Cimadomo D, et al. Management of women with an unexpected low ovarian response to gonadotropin. Front Endocrinol (Lausanne) 2019; 10: 387.
- Vaiarelli A, Cimadomo D, Argento C, et al. Double stimulation in the same ovarian cycle (DuoStim) is an intriguing strategy to improve oocyte yield and the number of competent embryos in a short timeframe. Minerva Ginecol 2019; 71: 372–376.
- Cimadomo D, Vaiarelli A, Colamaria S, et al. Luteal phase anovulatory follicles result in the production of competent oocytes: intra-patient paired case-control study comparing follicular versus luteal phase stimulations in the same ovarian cycle. Hum Reprod 2018; 33: 1442–1448.
- Tartagni M, Cicinelli MV, Baldini D, et al. Dehydroepiandrosterone decreases the age-related decline of the in vitro fertilization outcome in women younger than 40 years old. Reprod Biol Endocrinol 2015; 13: 18.






28 commenti
Hi there, I enjoy reading through your article post. I like to write a little comment to support you.
Hi, everything is going well here and ofcourse every one is sharing facts, that’s genuinely good, keep up writing.
Hi, Neat post. There’s a problem together with your site in web
explorer, would test this? IE still is the market chief
and a good component to people will leave out your magnificent writing because of this
problem.
You are so cool! I don’t think I’ve truly read through something like this
before. So great to discover somebody with genuine thoughts on this subject.
Seriously.. many thanks for starting this up. This site is
something that’s needed on the web, someone
with a bit of originality!
Hey! I’m at work browsing your blog from my new apple iphone!
Just wanted to say I love reading your blog and look forward to
all your posts! Carry on the fantastic work!
Howdy just wanted to give you a quick heads up and let you know a few of the pictures aren’t loading correctly.
I’m not sure why but I think its a linking issue.
I’ve tried it in two different web browsers and both show the same results.
Thank you for the good writeup. It in fact was a amusement account it.
Look advanced to more added agreeable from you!
By the way, how could we communicate?
I every time spent my half an hour to read this blog’s articles daily along
with a cup of coffee.
Everything is very open with a very clear description of
the issues. It was truly informative. Your website
is very useful. Thank you for sharing!
Hi it’s me, I am also visiting this web site daily, this site is in fact pleasant
and the visitors are genuinely sharing nice thoughts.
Hey there! Would you mind if I share your blog with my facebook group?
There’s a lot of folks that I think would really enjoy your content.
Please let me know. Thank you
Very great post. I just stumbled upon your blog and wanted to say that I have truly enjoyed surfing around your blog posts.
After all I’ll be subscribing on your rss feed and I hope you write once
more soon!
Hello, Neat post. There’s a problem along with your site in web explorer, may
check this? IE still is the market chief and a good component
to other folks will pass over your great writing because of this problem.
Have you ever considered writing an e-book or guest authoring on other blogs?
I have a blog based on the same information you discuss and would
really like to have you share some stories/information. I know my readers would enjoy your work.
If you are even remotely interested, feel free to
shoot me an e-mail.
Hello there! This is my first visit to your blog!
We are a group of volunteers and starting a new project
in a community in the same niche. Your blog provided us beneficial information to work on. You have done a outstanding job!
I do not even know the way I finished up here, however I assumed this publish was good.
I do not recognize who you’re however definitely you are going
to a well-known blogger when you are not already.
Cheers!
I’m gone to convey my little brother, that he should also go to see this weblog on regular basis to take updated from most recent reports.
Somebody necessarily lend a hand to make seriously articles I would state.
This is the first time I frequented your web page
and so far? I surprised with the research you made to make this
particular publish amazing. Wonderful process!
Hi! I’ve been reading your blog for some time now and
finally got the courage to go ahead and give you a shout out from Atascocita Tx!
Just wanted to tell you keep up the great work!
Greetings! I’ve been reading your weblog for a while
now and finally got the bravery to go ahead and give you a shout out
from Huffman Texas! Just wanted to mention keep up the good job!
What a stuff of un-ambiguity and preserveness of valuable
know-how on the topic of unpredicted emotions.
I got this web page from my buddy who shared with me
regarding this website and at the moment this time I am visiting this web site and reading very informative articles or reviews here.
This is a really good tip especially to those fresh
to the blogosphere. Brief but very precise information… Many
thanks for sharing this one. A must read post!
I read this paragraph completely on the topic of the difference of latest
and earlier technologies, it’s remarkable article.
I do not know if it’s just me or if perhaps everybody else experiencing
problems with your blog. It seems like some of the text within your posts are running off the screen. Can somebody else please comment and let me know if this is happening to them too?
This might be a problem with my browser because I’ve had this happen before.
Many thanks
Hello! Do you know if they make any plugins to protect against hackers?
I’m kinda paranoid about losing everything I’ve worked hard on. Any suggestions?
I like this publication, its very informative and help. Thank you very muych
Hello there! This is kind of off topic but I need some advice from an established blog.
Is it difficult to set up your own blog? I’m not
very techincal but I can figure things out pretty quick.
I’m thinking about setting up my own but I’m not sure where to begin. Do you have any
ideas or suggestions? Thanks