 so molti animali domestici, roditori e uccelli.
so molti animali domestici, roditori e uccelli.
I gatti sono considerati il serbatoio primitivo del Toxoplasma gondii perchè nel loro intestino avviene il ciclo riproduttivo sessuale, o primario, del Toxoplasma. I gatti si infettano cibandosi delle carni di roditori, uccelli e piccoli animali. Negli ultimi anni si è ridimensionata l’attenzione nei confronti del gatto come portatore della malattia, in particolare se si tratta di un gatto domestico, alimentato con prodotti in scatola e la cui lettiera è cambiata tutti i giorni (le cisti del parassita si schiudono dopo tre giorni a temperatura ambiente e alta umidità). Il vero serbatoio primario della
toxoplasmosi è invece rappresentato dai gatti randagi, che si infettano cacciando uccelli e topi contaminati, e che possono defecare nel terreno rilasciando oocisti di Toxoplasma che contaminano acqua, erba, foraggio, verdure e frutta che vengono ingeriti da suini, bovini, ovini e uccelli. Questi ultimi rappresentano gli ospiti intermedi del toxoplasma. Anche l’uomo rientrare fra gli ospiti intermedi potendosi contaminare direttamente con l’ingestione di frutta e verdure contaminate o a contatto con gatti o altri animali contaminati. Ma più frequentemente la contaminazione dell’uomo avviene con l’ingestione di carne cruda o poco cotta contenente oocisti di toxoplasma (30-70%).
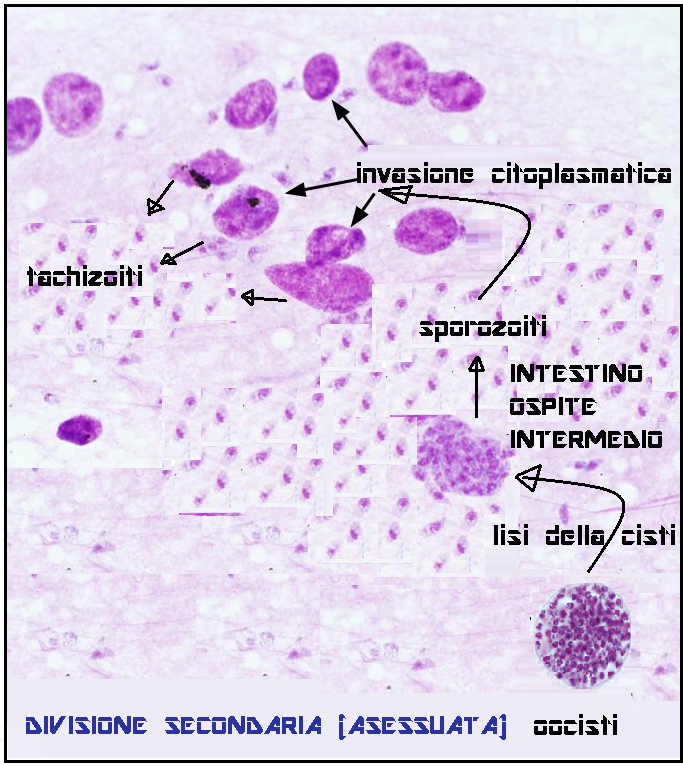
 La prima riproduzione del toxoplasma avviene nell’intestino tenue di gatto (ospite primario). I succhi gastrici ed intestinali del gatto corrodono la parete dell’oocisti incistidata nelle carni di roditori o uccellini e permettono agli sporozoiti contenuti nell’oocisti di fuoriuscire. Gli sporozoiti invadono le cellule intestinali del gatto dove si riproducono (riproduzione sessuata) fino a far scoppiare le cellule stesse. Gli spororozoiti quindi si riorganizzano in nuove e più numerose oocisti che vengono espulse con le feci del gatto contaminando terreno, acqua, erba, foraggio, frutta e verdure.
La prima riproduzione del toxoplasma avviene nell’intestino tenue di gatto (ospite primario). I succhi gastrici ed intestinali del gatto corrodono la parete dell’oocisti incistidata nelle carni di roditori o uccellini e permettono agli sporozoiti contenuti nell’oocisti di fuoriuscire. Gli sporozoiti invadono le cellule intestinali del gatto dove si riproducono (riproduzione sessuata) fino a far scoppiare le cellule stesse. Gli spororozoiti quindi si riorganizzano in nuove e più numerose oocisti che vengono espulse con le feci del gatto contaminando terreno, acqua, erba, foraggio, frutta e verdure.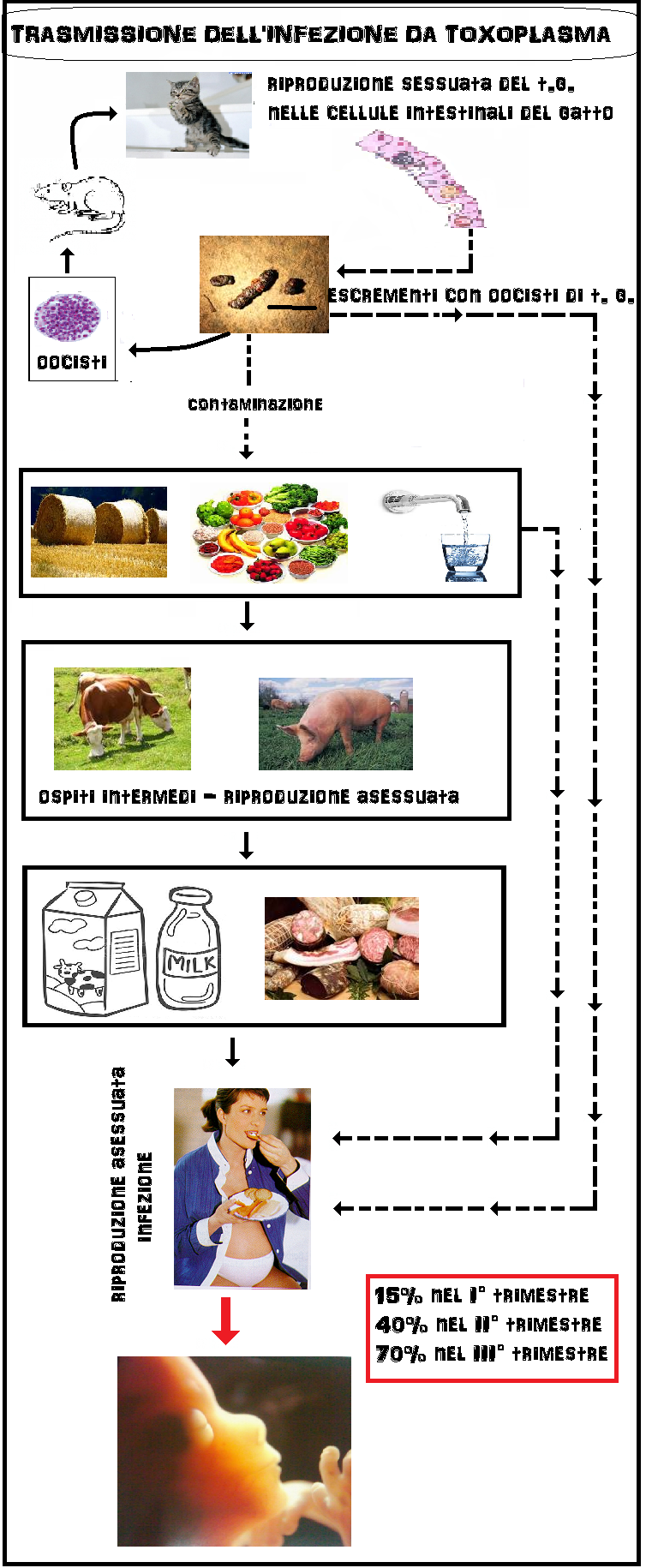
 malformazioni fetali. Anche la josamicina, come la spiramicina è escreta nel latte materno. La posologia abituale è di 1-2 g/die in 2 o più assunzioni. In caso di infezioni più gravi il dosaggio può essere aumentato sino a 3 g/die.
malformazioni fetali. Anche la josamicina, come la spiramicina è escreta nel latte materno. La posologia abituale è di 1-2 g/die in 2 o più assunzioni. In caso di infezioni più gravi il dosaggio può essere aumentato sino a 3 g/die.
- test diagnostici diretti: coltura cellulare, amplificazione del genoma (PCR, Polymerase Chain Reaction);
- test diagnostici indiretti: IFA (immunofluorescenza indiretta), ELISA (IgA, IgM, IgG), ISAGA (immunoassorbimento per IgM), Western-blot, test di avidità per le IgG.
TERAPIA: Il trattamento di elezione nella toxoplasmosi congenita rimane l’associazione pirimetamina (Daraprim® cpr 25 mg) e sulfadiazina (Sulfadiazina® cpr 500 mg), prese individualmente o già confezionate in associazione (Metakelfin® cpr 25 mg + 500 mg). La terapia dovrebbe essere intrapresa con lo scopo di prevenire la notevole incidenza di sequele tardive segnalate nei bambini che ricevono trattamento inadeguato o nessun trattamento. Entrambi i farmaci sono efficaci sulla forma attiva del ciclo vitale del parassita (tachizoita); il razionale della terapia durante l’infezione acuta è appunto quello di distruggere i tachizoiti e prevenirne la trasformazione in nuove cisti, su cui questi farmaci non sono attivi. La pirimetamina e la sulfadiazina esercitano un effetto sinergico contro il T. gondii interferendo sul suo metabolismo dei folati in due punti diversi. L’attività combinata dei due farmaci è di otto volte superiore a quella ottenibile con la somma dei singoli effetti. Grazie a questa azione sinergica sulla via di sintesi dei folati, è possibile ridurre il dosaggio della pirimetamina e quindi moderarne gli effetti ematotossici. La sulfadiazina si somministra al dosaggio di 100 mg/kg/die in 2 somministrazioni giornaliere per 12 mesi di terapia. La tollerabilità di entrambi i farmaci è buona e non sono segnalati effetti di tossicità, né discrasie ematologiche o patologie maligne a insorgenza tardiva, nei neonati che siano stati trattati a lungo con pirimetamina e sulfadiazina. Posologia compresse (combinazione 25 mg di pirimetamina e 500 mg di sulfadiazina), per os, in monosomministrazione:
- <1 anno di età: 0,25 cp/die
- 1-3 anni: ½ cp/die
- 4-8 anni: 1 cp/die in
- 9-14 anni: 2 cp/die
-
Dubey JP, Lindsay DS, Speer CA: Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev 1998;11:267-299
-
Ajzenberg D, Cogné N, Paris L. Genotype of 86 Toxoplasma gondii isolates associated with human congenital toxoplasmosis, and correlation with clinical findings. J Infect Dis. 2009;186:684-89.
- Pollina S.: “Toxoplasmosi in gravidanza”. Sito internet 2005.
- Conti F.: Infezioni in gravidanza; Artemisia News, Aprile 2003 23.
-
Wong S, Remington JS: Toxoplasmosis in pregnancy. Clin Infect Dis 1994;18:853-862
- Boyer K et al. Risk factors for Toxoplasma gondii infection in mothers of infants with congenital toxoplasmosis: Implications for prenatal management and screening. Am J Obstet Gynecol. 2005 Feb;192(2):564-71
-
Dubey JP, Hill DE, Jones JL, et al. Prevalence of viable Toxoplasma gondii in beef, chicken, and pork from retail meat stores in the United States: risk assessment to consumers. J Parasitol 2005;91:1082–93
-
de Moura L, Bahia-Oliveira LM, Wada MY et al. Waterborne toxoplasmosis, Brazil, from field to gene Emerg Infect Dis 2006;12: 326-329
-
Bowie WR, King AS, Werker DH , et al. Outbreak of toxoplasmosis associated with municipal drinking water Lancet 1997:350:173-177
-
Bahia-Oliveira LM, Jones JL, Azevedo-Silva J, Alves CC, Orefice F, Addiss DG. Highly endemic, waterborne toxoplasmosis in north Rio de Janeiro state, Brazil. Emerg Infect Dis 2003;9:55-62
-
Lin YL, Liao YS, Liao LR, Chen FN, Kuo HM, He S. Seroprevalence and sources of Toxoplasma infection among indigenous and immigrant pregnant women in Taiwan. Parasitol Res 2008; published online Mar 2008
-
Mitchell CD, Erlich SS, Mastrucci MT, Hutto SC, Parks WP, Scott G: Congenital toxoplasmosis occurring in infants perinatally infected with human immunodeficiency virus 1. Pediatr Infect Dis J 1990;9:512-518
-
Dunn D, Wallon M, Peyron F, Petersen E, Peckham C, Gilbert R. Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counselling. Lancet 1999;353:1829-1833
- Centers for Disease Control and Prevention. Toxoplasmosis: Pregnant Women. [Cited 2010 October 11]. Available at URL: http://www.cdc.gov/toxoplasmosis/pregnant.html.
- Kieffer F, Wallon M, Garcia P, Thulliez P, Peyron F, Franck J: Risk factors for retinochoroiditis during the first 2 years of life in infants with treated congenital toxoplasmosis. Pediatr Infect Dis J 2008;27:27-32
-
Garweg JG, Scherrer J, Wallon M, Kodjikian L, Peyron F: Reactivation of ocular toxoplasmosis during pregnancy. BJOG 2005;112:241:-242
- Cook AJC, et al. 2000. Sources of toxoplasmosis in pregnant women. BMJ 321:142-147.
-
Bretagne S: Molecular diagnostics in clinical parasitology and mycology: limits of the current polymerase chain reaction (PCR) assays and interest of the real-time PCR assays. Clin Microbiol Infect 2003;9:505-511
-
Montoya JG, Liesenfeld O, Kinney S, Press C, Remington JS. VIDAS test for avidity of Toxoplasma-specific immunoglobulin G for confirmatory testing of pregnant women. J Clin Microbiol 2002; 40:2504-8
-
Lappalainen M, Koskela P, Koskiniemi M et al.: Toxoplasmosis acquired during pregnancy: improved serodiagnosis based on avidity of IgG. J Infect Dis 1993:167:691-697
-
Montoya JG, Huffman HB, Remington JS: Evaluation of the immunoglobulin G avidity test for diagnosis of toxoplasmic lymphadenopathy. J Clin Microbiol 2004;42:4627-4631
-
Romand S, Wallon M, Franck J, Thulliez P, Peyron F, Dumon H: Prenatal diagnosis using polymerase chain reaction on amniotic fluid for congenital toxoplasmosis. Obstet Gynecol 2001;97:296-300
-
Roberts A, Hedman K, Luyasu V, et al.: Multicenter evaluation of strategies for serodiagnosis of primary infection with Toxoplasma gondii. Eur J Clin Microbiol Infect Dis 2001;20:467-474
-
Lappalainen M, Hedman K. Serodiagnosis of toxoplasmosis: the impact of measurement of IgG avidity.Ann Ist Super Sanita 2004;40:81-88
-
Liesenfeld O, Montoya JG, Tathineni NJ , et al. Confirmatory serologic testing for acute toxoplasmosis and rate of induced abortions among women reported to have positive Toxoplasma immunoglobulin M antibody titers. Am J Obstet Gynecol 2001;184:140-145
- Gollub EL, Leroy V, Gilbert R, Chene G, Wallon M: Effectiveness of health education on Toxoplasma-related knowledge, behaviour, and risk of seroconversion in pregnancy. Eur J Obstet Gynecol Reprod Biol 2008;136:137-145
- Daffos F, et al. 1988. Prenatal management of 746 pregnancies at risk for congenital toxoplasmosis. N Engl J Med 318(5):271-275.
- Dubey JP and Jones JL. 2008. Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol 38:1257-1278.
- Hide G, et al. 2009. Evidence for high levels of vertical transmission in Toxoplasma gondii. Parasitol 136:1877-1885. 10. McLeod, R., et al. “Outcome of Treatment for Congenital Toxoplasmosis”. 1981-2004: The National Collaborative Chicago-Based, Congenital Toxoplasmosis Study. Clinical InEJfectious Diseases, volume 15, number 42, May 15, 2006, pages 1383-1394.
- Guerina N, Hsu W, Meissner H et al: Neonatal serologic screening and early treatment for congenital Toxoplasma gondii infection. N Engl J Med 1994;330:1858-1863)
- Fricker-Hidalgo H, Brenier-Pinchart MP, Schaal JP, Equy V, Bost-Bru C, Pelloux H: Value of Toxoplasma gondii detection in one hundred thirty-three placentas for the diagnosis of congenital toxoplasmosis. Pediatr Infect Dis J 2007;26:845-846
- Goldstein EJC, Montoya JG, Remington JS: Management of Toxoplasma gondiiInfection during Pregnancy. Clin Inf Diseases.;2008;47,4:554-566
-
Thulliez P. Commentary: efficacy of prenatal treatment for toxoplasmosis: a possibility that cannot be ruled out. Int J Epidemiol 2001;30:1315-1316
-
Gilbert R, Gras L: European Multicentre Study on Congenital Toxoplasmosis. Effect of timing and type of treatment on the risk of mother to child transmission of Toxoplasma gondii. BJOG 2003;110:112-120
-
Thiebaut R, Leproust S, Chene G, Gilbert R: Effectiveness of prenatal treatment for congenital toxoplasmosis: a meta-analysis of individual patients’ data. Lancet 2007;369:115-122
-
Berrebi A, Bardou M, Bessieres MH , et al. Outcome for children infected with congenital toxoplasmosis in the first trimester and with normal ultrasound findings: a study of 36 cases. Eur J Obstet Gynecol Reprod Biol 2007;135:53-57
-
Foulon W, Naessens A, Lauwers S, De Meuter F, Amy JJ: Impact of primary prevention on the incidence of toxoplasmosis during pregnancy. Obstet Gynecol 1988;72:363-366
-
Forestier F: Les foetopathies infectieuses: prevention, diagnostic prenatal, attitude pratique. Presse Med1991:20:1448-1454
-
Remington JS, McLeod R, Thuilliez P, Desmonts G. Remington JS, Klein JO, Wilson CB, Baker C. Toxoplasmosis Infectious diseases of the fetus and newborn infant. 2006 6th ed. Philadelphia Elsevier Saunders (pg. 947-1091)
-
Schmidt DR, Hogh B, Andersen O, Fuchs J, Fledelius H, Petersen E The national neonatal screening programme for congenital toxoplasmosis in Denmark: results from the initial four years, 1999–2002 Arch Dis Child 2006;91:661-665
-
Bollani L, Stronati M: Il neonato con toxoplasmosi congenita: clinica, terapia e follow-up. J Ped Neonatal Indiv Med 2014
-
Thiébaut R, Leproust S, Chêne G, Gilbert R. Effectiveness of prenatal treatment for congenital toxoplasmosis: a meta-analysis of individual patients’ data. Lancet. 2007;369:115-22
-
Ajzenberg D, Cogné N, Paris L. Genotype of 86 Toxoplasma gondii isolates associated with human congenital toxoplasmosis, and correlation with clinical findings. J Infect Dis. 2009;186:684-89.
-
Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965-76.
-
Wallon M, Kodjikian L, Binquet C, Garweg J, Fleury J, Quantin C, Peyron F. Long term ocular prognosis in 327 children with congenital toxoplasmosis. Pediatrics. 2004;113:1567-72.
-
Petersen E, Schmitdt DR. Sulfadiazine and pyrimethamine in postnatal treatment of congenital toxoplasmosis: what are the options? Expert Rev Anti Infect Ther. 2008;1(1):175-83.
-
Schmidt DR, Hogh B, Andersen O, Hansen SH, Dalhoff K, Petersen E. Treatment of infants with congenital toxoplasmosis: tolerability and plasma concentrations of sulfadiazine and pyrimetamine. Eur J Pediatr. 2006;165:19-25
-
Derouin F, Almadany R, Chau F, Rouveix B, Pocidalo JJ. Synergistic activity of azithromycin and pyrimetamine or sulfadiazine in acute experimental toxoplasmosis. Antimicrob Agents Chemother. 1992;36:997-1001
-
Strausbaugh LJ, Dilworth JA, Gwaltney JM, Sande MA, In vitro susceptibility studies with josamycin and erythromycin, in Antimicrob. Agents Chemother., vol. 9, nº 3, marzo 1976, pp. 546–8,
-
Couvreur J, Desmonts G, Thulliez P: Prophylaxis of congenital toxoplasmosis: effects of spiramycin on placental infection. J Antimicrob Chemother 1988;22:193-200


1 commento
I think you have noted some very interesting details , thankyou for the post.