Ultimo aggiornamento 2020-10-29 13:12:29
Il Gn-RH (Gonadotropin Releasing Hormone) è un ormone secreto dai neuroni migrati durante la vita embrionale dal placode olfattivo ai nuclei ipotalamici medio-basali. La mancata o parziale migrazione di tali neuroni durante lo sviluppo fetale è responsabile della S. di Kallmann, dell’ipogonadismo ipogonadotropo e dell’amenorrea primaria ipotalamica (1-4).
Il Gn-RH ha una struttura peptidica composta da 10 aminoacidi: ac. glutammico, Serina, glicina, Istidina, Prolina, Tirosina, Leucina, Lisina, Isoleucina, Triptofano e Fenil-alanina.
I nuclei ipotalamici interessati alla secrezione di Gn-RH sono il n. arcuato, il n. sovraottico, il n. medio-basale e il n. paraventricolare posizionati nell’ipotalamo medio-basale tra il III° ventricolo e l’eminenza mediana in prossimità del circolo ipotalamo-ipofisario dove viene riversato il Gn-RH e trasportato all’adenoipofisi senza necessità di attraversare la barriera emato-cerebrale 2-4).
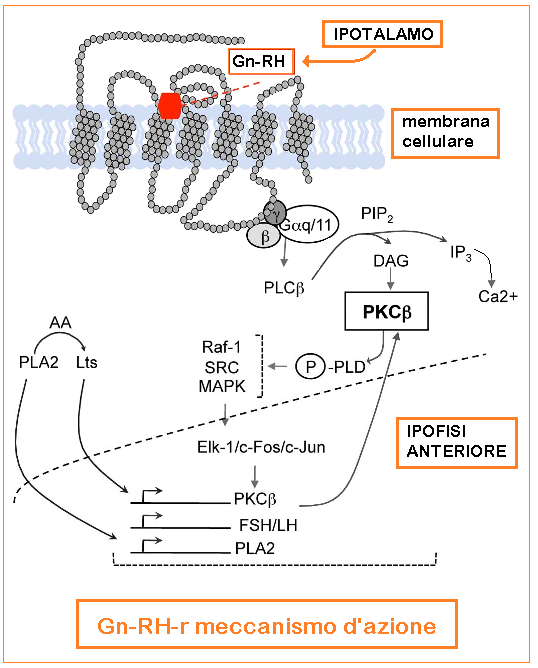
Meccanismo d’azione del Gn-RH: la secrezione di Gn-RH è soppressa durante l’infanzia, inizia ad aumentare alla pubertà e termina nel periodo post-menopausale. La secrezione è di tipo pulsatile (1 pulse ogni 60-90 minuti) e agisce sulle cellule basofile dell’adenoipofisi tramite il complesso costituito recettore specifico accoppiato a proteina G (GPCR) → fosfolipasi C (PLC) → Ca++ → Protein-chinasi C → rilascio ipofisario FSH e soprattutto di LH (5-8).
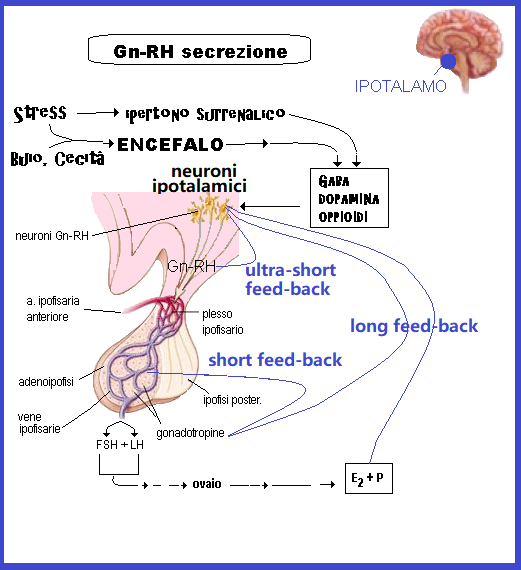
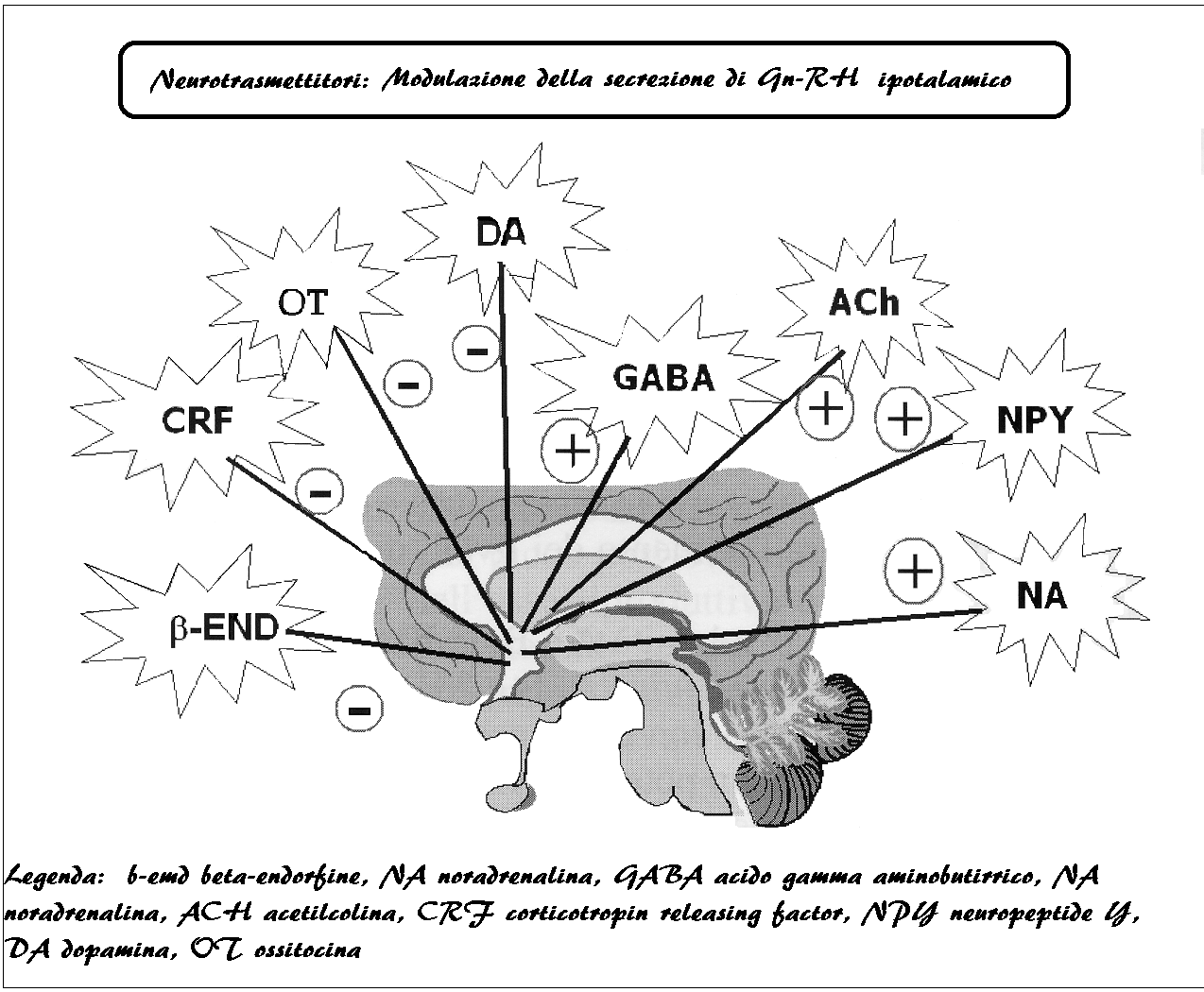
L’ampiezza dei pulses di Gn-RH è regolato con sistema feed-back lungo dalle concentrazioni ematiche di FSH ed LH e con feed-back corto dalla stessa produzione di Gn-RH. Sulla normale secrezione di Gn-RH inoltre agiscono altri numerosissimi fattori: integrità strutturale del gene del Gn-RH, stato nutritivo della paziente che deve essere dotata di un minimo di massa grassa, oppioidi, GABA, dopamina (2).
Anche la presenza o meno della luce naturale e la lunghezza del giorno influiscono sulla secrezione di Gn-RH e sull’attività dell’asse ipotalamo-ipofisario; infatti nei paesi nordici l’indice di concepimento è inferiore a quello dei paesi mediterranei e tropicali e nelle pazienti non-vedenti si presentano più frequentemente alterazioni funzionali dell’asse ipotalamo-ipofisi-ovarico (9-17).
Nelle pazienti PCOS aumenta l’ampiezza dei picchi con conseguente aumentata secrezione di LH (non di FSH). Nelle pazienti con amenorrea ipotalamica la frequenza dei picchi è molto aumentata e l’ampiezza diminuita (18-22).
La prolattina svolge un ruolo inibente sulla secrezione di Gn-RH.
Variazioni della frequenza e dell’ampiezza della secrezione del GnRH sono alla base dell’induzione della pubertà e del meccanismo che porta all’ ovulazione nella donna. Durante il ciclo mestruale, la secrezione di Gn-RH aumenta in fase follicolare fino a raggiungere l’acme in fase immediatamente pre-ovulatoria (23-30).
I neuroni si spingono giù fino all’eminenza mediana, infundibolo e peduncolo superiore dell’ipofisi dove sfociano nei vasi portali che trasportano il Gn-RH alle cellule basofile dell’adenoipofisi.
Il n. arcuato è costituito da 1000-3000 cellule che sono capaci di una secrezione pulsatile di Gn-RH (1 pulse ogni 60-90 minuti).
USI CLINICI:
La somministrazione pulsatile di Gn-RH, mediante pompa da infusione, è utilizzata per stimolare la secrezione di di FSH ed LH, per confermare la diagnosi e terapia di ipogonadismo ipogonadotropo, amenorrea ipotalamica e anovulazione ipotalamica (31-35),
La somministrazione im/sc di Analoghi (Gn-RH-a), ormoni di sintesi simili al Gn-RH, in preparazione depot comporta inibizione della secrezione di LH/FSH (dopo un flare-up iniziale) e riduzione dell’espressione del recettore. Viene utilizzata per la terapia di Pubertà precoce centrale, Carcinomi prostatico e mammario, iperplasia endometriale steroido-dipendente, Leiomiomi uterini, Endometriosi, Sindrome dell’ovaio policistico, Irsutismo, Turbe sessuali. Alcuni nomi commerciali di Gn-RH-a:
- leuprorelina (Lupron, Eligard)
- buserelina (Suprefact, Suprecor)
- nafarelina (Synarel)
- istrelina (Supprelin LA, Vantas)
- goserelina (Zoladex)
- deslorelina (Suprelorin, Ovuplant)
- triptorelina (Gonapeptyl, Decapeptyl)
(Decapeptyl® 0.1 mg/ml fl sc 1 ml (quotidiano), Decapeptyl fl 2 ml im 3,75 mg (mensile), Decapeptyl fl im 11,25 mg (trimestrale); Gonapeptyl depot® 3,75 mg, Zoladex® fl sc 3,60 mg (mensile), Zoladex 10,8 mg (trimestrale); Suprefact® fl im 5,5 ml 1 mg/ml (mensile), Suprefact® spray nasale 10 mg soluzione 0,1% una spruzzata = 0.1 mg per narice per 3-6 volte al dì)
Effetti avversi analoghi GnRH:
- Iniziale iperstimolazione gonadotropinica (flare-up)
- Ipogonadismo
- Vampate di calore
- Secchezza e atrofia vaginale
- Impotenza
- Osteoporosi
Antagonisti recettoriali del Gn-RH (Ganirelix, Cetrorelix): Stessi usi clinici degli analoghi però non inducono il flare up iniziale.
Test al Gn-RH
Il test può rilevare la presenza di ipogonadismo primario o secondario. Si determina la concentrazione ematica basale di LH. Di seguito vengono somministrati per via endovenosa 200 mg di LHRH sintetico, l’analogo del GnRH naturale. Prelievi vengono effettuati dopo 15-30-60-90-120 minuti (36-58).
Normalmente l’LH aumenta del 30-100% dopo 15-30 minuti dalla somministrazione di Gn-RH mentre l’FSH presenta un aumento del 10-50% dopo 30-60 minuti.
Alterazioni gonadotropiniche:
- Deficit ovarico: aumento di LH e FSH
- Deficit ipotalamico: LH e FSH ridotti
- Deficit ipofisario: LH in aumento e FSH ridotto
- Iperprolattinemia: LH e FHS leggermente aumentati
- Pubertà precoce: aumento di FSH e LH
- PCOS: LH aumentato ed FSH ridotto o inalterato
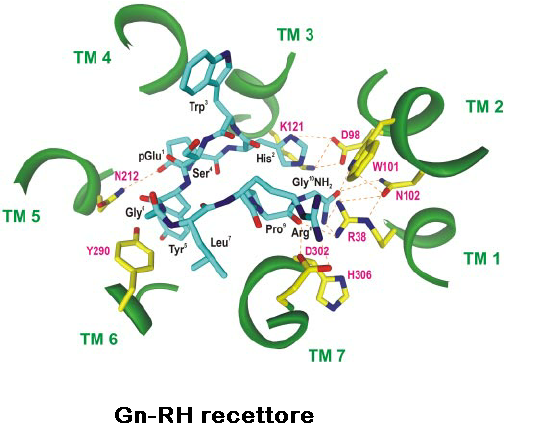
Gn-RH recettori: Il Gn-RH regola l’endocrinologia della riproduzione legandosi e stimolando specifici recettori posti sulle cellule gonadotrope dell’adenoipofisi così che esse possano secernere FSH ed LH.
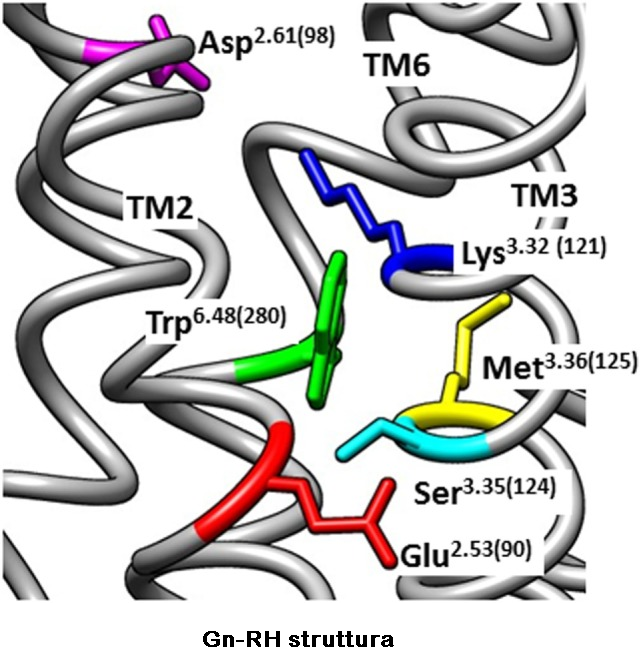
l recettori Gn-RH mancano di una coda carbossi-terminale citoplasmatica ma possiedono una sequenza aminoacidica caratteristica dei recettori accoppiati a proteine di classe A, G rodopsina-simili (GPCR). I ligandi si legano a superfici extracellulari variabili, mentre le sette eliche α-membrana trasmettono il segnale di attivazione alla superficie del recettore citoplasmatico, che lega e attiva le proteine G eterotrimeriche. Quaranta interazioni non covalenti che collegano residui topologicamente equivalenti in diverse eliche transmembrana (TM) sono conservate in strutture di classe A GPCR, indipendentemente dallo stato di attivazione. I contatti interhelici indipendenti dalla conformazione rappresentano una struttura proteica conservata dei recettori e la loro importanza nella struttura dei recettori del GnRH è supportata dalla ridotta espressione dei recettori con mutazioni dei residui nella rete.
Le mutazioni del recettore GnRH associate a ritardo della pubertà e ipogonadismo
ipogonadotropico congenito, Questo ruolo centrale nella regolazione della riproduzione ha reso il GnRH-r un bersaglio per il trattamento dell’infertilità e dell’iperplasia steroidea-dipendente, tra cui fibromi uterini, endometriosi e cancro prostatico, dove la produzione di steroidi gonadici può essere ridotta dalla somministrazione di antagonisti del GnRH o da alte dosi di Agonisti del GnRH, che riducono l’espressione del recettore.
La metà delle circa 250 interazioni intramolecolari nei GPCR differiscono tra le strutture inattive e attive. I contatti interelastici specifici per la conformazione dipendono dagli amminoacidi che cambiano partner durante l’attivazione. Conservati contatti specifici di conformazione inattiva impediscono l’attivazione del recettore stabilizzando la prossimità delle eliche TM 3 e 6 e un sito di legame alle proteine G chiuso (60-90).
Bibliografia:
- C Kubili-Garfias: Electronic structure of gonadotropin-releasing hormone (GnRH): a semiempirical study. Journal of Molecular Structure: THEOCHEM Volume 529, Issues 1–3, 8 September 2000, Pages 203-208
- Manilall A, Stander BA, Madziva MT, Millar RP, Flanagan CA. Mol Cell Endocrinol. 2019 Feb 5; 481:53-61. Epub 2018 Nov 23.
- Bhattacharya S, Hall SE, Vaidehi N. J Mol Biol. 2008 Oct 3; 382(2):539-55. Epub 2008 Jul 7.
- Lu ZL, Gallagher R, Sellar R, Coetsee M, Millar RP. J Biol Chem. 2005 Aug 19; 280(33):29796-803. Epub 2005 Jun 20.
- Rovati GE, Capra V, Neubig RR. Mol Pharmacol. 2007 Apr; 71(4):959-64. Epub 2006 Dec 27.
- Colleen A. Flanagan and Ashmeetha Manilall: Gonadotropin-Releasing Hormone (GnRH) Receptor Structure and GnRH Binding Front Endocrinol (Lausanne). 2017; 8: 274.
- Bedecarrats GY, Kaiser UB. Mutations in the human gonadotropin-releasing hormone receptor: insights into receptor biology and function. Semin Reprod Med (2007) 25(5):368–78.10.1055/s-2007-984743
- Brioude F, Bouligand J, Trabado S, Francou B, Salenave S, Kamenicky P, et al. Non-syndromic congenital hypogonadotropic hypogonadism: clinical presentation and genotype-phenotype relationships. Eur J Endocrinol (2010) 162(5):835–51.10.1530/EJE-10-0083
- Naor Z. Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor. Front Neuroendocrinol (2009) 30(1):10–29.10.1016/j.yfrne.2008.07.001
- Schally AV, Block NL, Rick FG. Discovery of LHRH and development of LHRH analogs for prostate cancer treatment. Prostate (2017) 77(9):1036–54.10.1002/pros.23360
- Struthers RS, Nicholls AJ, Grundy J, Chen T, Jimenez R, Yen SS, et al. Suppression of gonadotropins and estradiol in premenopausal women by oral administration of the nonpeptide gonadotropin-releasing hormone antagonist elagolix. J Clin Endocrinol Metab (2009) 94(2):545–51.10.1210/jc.2008-1695
- Bliss SP, Navratil AM, Xie J, Roberson MS. GnRH signaling, the gonadotrope and endocrine control of fertility. Front Neuroendocrinol (2010) 31(3):322–40.
- Tsutsumi R, Mistry D, Webster NJ. Signaling responses to pulsatile gonadotropin-releasing hormone in LbetaT2 gonadotrope cells. J Biol Chem (2010) 285(26):20262–72.
- Choi SG, Jia J, Pfeffer RL, Sealfon SC. G proteins and autocrine signaling differentially regulate gonadotropin subunit expression in pituitary gonadotrope. J Biol Chem (2012) 287(25):21550–60.
- Naor Z, Huhtaniemi I. Interactions of the GnRH receptor with heterotrimeric G proteins. Front Neuroendocrinol (2013) 34(2):88–94.10.1016/j.yfrne.2012.11.001
- White CD, Coetsee M, Morgan K, Flanagan CA, Millar RP, Lu ZL. A crucial role for Galphaq/11, but not Galphai/o or Galphas, in gonadotropin-releasing hormone receptor-mediated cell growth inhibition. Mol Endocrinol (2008) 22(11):2520–30.10.1210/me.2008-0122
- Grosse R, Schmid A, Schoneberg T, Herrlich A, Muhn P, Schultz G, et al. Gonadotropin-releasing hormone receptor initiates multiple signaling pathways by exclusively coupling to G(q/11) proteins. J Biol Chem (2000) 275(13):9193–200.
- Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-releasing hormone receptors. Endocr Rev (2004) 25(2):235–75.
- Munk C, Isberg V, Mordalski S, Harpsoe K, Rataj K, Hauser AS, et al. GPCRdb: the G protein-coupled receptor database – an introduction. Br J Pharmacol (2016) 173(14):2195–207.
- Isberg V, de Graaf C, Bortolato A, Cherezov V, Katritch V, Marshall FH, et al. Generic GPCR residue numbers – aligning topology maps while minding the gaps. Trends Pharmacol Sci (2015) 36(1):22–31.
- Mahoney JP, Sunahara RK. Mechanistic insights into GPCR-G protein interactions. Curr Opin Struct Biol (2016) 41:247–54.Venkatakrishnan AJ, Deupi X, Lebon G, Heydenreich FM, Flock T, Miljus T, et al. Diverse activation pathways in class A GPCRs converge near the G-protein-coupling region. Nature (2016) 536(7617):484–7.
- Tesmer JJ. Hitchhiking on the heptahelical highway: structure and function of 7TM receptor complexes. Nat Rev Mol Cell Biol (2016) 17(7):439–50.
- Cvicek V, Goddard WA, III, Abrol R. Structure-based sequence alignment of the transmembrane domains of all human GPCRs: phylogenetic, structural and functional implications. PLoS Comput Biol(2016) 12(3):e1004805.10.1371/journal.pcbi.1004805
- Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature (2013) 494(7436):185–94.10.1038/nature11896
- Ballesteros JA, Weinstein W. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci(1995) 25:366–428.
- Huang W, Manglik A, Venkatakrishnan AJ, Laeremans T, Feinberg EN, Sanborn AL, et al. Structural insights into micro-opioid receptor activation. Nature (2015) 524(7565):315–21.
- Flanagan CA, Zhou W, Chi L, Yuen T, Rodic V, Robertson D, et al. The functional microdomain in transmembrane helices 2 and 7 regulates expression, activation, and coupling pathways of the gonadotropin-releasing hormone receptor. J Biol Chem (1999) 274(41):28880–6.
- Katritch V, Fenalti G, Abola EE, Roth BL, Cherezov V, Stevens RC. Allosteric sodium: a key co-factor in class A GPCR signaling. Trends Biochem Sci (2014) 39(5):233–44.
- Zhou W, Flanagan C, Ballesteros JA, Konvicka K, Davidson JS, Weinstein H, et al. A reciprocal mutation supports helix 2 and helix 7 proximity in the gonadotropin-releasing hormone receptor. Mol Pharmacol (1994) 45(2):165–70.
- Trzaskowski B, Latek D, Yuan S, Ghoshdastider U, Debinski A, Filipek S. Action of molecular switches in GPCRs-theoretical and experimental studies. Curr Med Chem (2012) 19(8):1090–109.
- Carpenter B, Nehme R, Warne T, Leslie AG, Tate CG. Structure of the adenosine A(2A) receptor bound to an engineered G protein. Nature (2016) 536(7614):104–7.
- Goncalves JA, South K, Ahuja S, Zaitseva E, Opefi CA, Eilers M, et al. Highly conserved tyrosine stabilizes the active state of rhodopsin. Proc Natl Acad Sci U S A (2010) 107(46):19861–6.
- Ballesteros J, Kitanovic S, Guarnieri F, Davies P, Fromme BJ, Konvicka K, et al. Functional microdomains in G-protein-coupled receptors. The conserved arginine-cage motif in the gonadotropin-releasing hormone receptor. J Biol Chem (1998) 273(17):10445–53.
- Topaloglu AK, Lu ZL, Farooqi IS, Mungan NO, Yuksel B, O’Rahilly S, et al. Molecular genetic analysis of normosmic hypogonadotropic hypogonadism in a Turkish population: identification and detailed functional characterization of a novel mutation in the gonadotropin-releasing hormone receptor gene. Neuroendocrinology (2007) 84(5):301–8.
- Deupi X, Standfuss J. Structural insights into agonist-induced activation of G-protein-coupled receptors. Curr Opin Struct Biol (2011) 21(4):541–51.
- Deupi X. Relevance of rhodopsin studies for GPCR activation. Biochim Biophys Acta (2014) 1837(5):674–82.
- Standfuss J, Edwards PC, D’Antona A, Fransen M, Xie G, Oprian DD, et al. The structural basis of agonist-induced activation in constitutively active rhodopsin. Nature (2011) 471(7340):656–60.
- Conn PM, Leanos-Miranda A, Janovick JA. Protein origami: therapeutic rescue of misfolded geneproducts. Mol Interv (2002) 2(5):308–16.
- Janovick JA, Maya-Nunez G, Conn PM. Rescue of hypogonadotropic hypogonadism-causing and manufactured GnRH receptor mutants by a specific protein-folding template: misrouted proteins as a novel disease etiology and therapeutic target. J Clin Endocrinol Metab (2002) 87(7):3255–62.
- Tello JA, Newton CL, Bouligand J, Guiochon-Mantel A, Millar RP, Young J. Congenital hypogonadotropic hypogonadism due to GnRH receptor mutations in three brothers reveal sites affecting conformation and coupling. PLoS One (2012) 7(6):e38456.
- Betz SF, Reinhart GJ, Lio FM, Chen C, Struthers RS. Overlapping, nonidentical binding sites of different classes of nonpeptide antagonists for the human gonadotropin-releasing hormone receptor. J Med Chem (2006) 49(2):637–47.
- Coetsee M, Gallagher R, Millar R, Flanagan C, Lu ZL. Role of Trp280(6.48) in the Gonadotropin-releasing hormone (GnRH) receptor. Abstrct, BioScience2006. Glasgow, UK: (2006).
- Arora KK, Cheng Z, Catt KJ. Dependence of agonist activation on an aromatic moiety in the DPLIY motif of the gonadotropin-releasing hormone receptor. Mol Endocrinol (1996)
- Roch GJ, Busby ER, Sherwood NM. GnRH receptors and peptides: skating backward. Gen Comp Endocrinol (2014) 209:118–34.
- Flanagan CA, Chen CC, Coetsee M, Mamputha S, Whitlock KE, Bredenkamp N, et al. Expression, structure, function, and evolution of gonadotropin-releasing hormone (GnRH) receptors GnRH-R1SHS and GnRH-R2PEY in the teleost, Astatotilapia burtoni. Endocrinology (2007) 148(10):5060–71.
- Kah O, Lethimonier C, Somoza G, Guilgur LG, Vaillant C, Lareyre JJ. GnRH and GnRH receptors in metazoa: a historical, comparative, and evolutive perspective. Gen Comp Endocrinol (2007) 153(1–3):346–64.
- Sefideh FA, Moon MJ, Yun S, Hong SI, Hwang JI, Seong JY. Local duplication of gonadotropin-releasing hormone (GnRH) receptor before two rounds of whole genome duplication and origin of the mammalian GnRH receptor. PLoS One (2014) 9(2):e87901.
- Chang JP, Pemberton JG. Comparative aspects of GnRH-stimulated signal transduction in the vertebrate pituitary – contributions from teleost model systems. Mol Cell Endocrinol(2017).
- Plachetzki DC, Tsai PS, Kavanaugh SI, Sower SA. Ancient origins of metazoan gonadotropin-releasing hormone and their receptors revealed by phylogenomic analyses. Gen Comp Endocrinol (2016) 234:10–9.
- Williams BL, Akazome Y, Oka Y, Eisthen HL. Dynamic evolution of the GnRH receptor gene family in vertebrates. BMC Evol Biol (2014) 14:215.
- Leanos-Miranda A, Janovick JA, Conn PM. Receptor-misrouting: an unexpectedly prevalent and rescuable etiology in gonadotropin-releasing hormone receptor-mediated hypogonadotropic hypogonadism. J Clin Endocrinol Metab (2002) 87(10):4825–8.
- Janovick JA, Stewart MD, Jacob D, Martin LD, Deng JM, Stewart CA, et al. Restoration of testis function in hypogonadotropic hypogonadal mice harboring a misfolded GnRHR mutant by pharmacoperone drug therapy. Proc Natl Acad Sci U S A (2013) 110(52):21030–5.
- Mirzadegan T, Benko G, Filipek S, Palczewski K. Sequence analyses of G-protein-coupled receptors: similarities to rhodopsin. Biochemistry (2003) 42(10):2759–67.
- Blankenship E, Vahedi-Faridi A, Lodowski DT. The high-resolution structure of activated opsin reveals a conserved solvent network in the transmembrane region essential for activation. Structure (2015) 23(12):2358–64.
- DeVree BT, Mahoney JP, Velez-Ruiz GA, Rasmussen SG, Kuszak AJ, Edwald E, et al. Allosteric coupling from G protein to the agonist-binding pocket in GPCRs. Nature (2016) 535(7610):182–6.
- Samama P, Cotecchia S, Costa T, Lefkowitz RJ. A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J Biol Chem (1993) 268(7):4625–36.
- Nygaard R, Zou Y, Dror RO, Mildorf TJ, Arlow DH, Manglik A, et al. The dynamic process of beta(2)-adrenergic receptor activation. Cell (2013) 152(3):532–42.
- Kenakin T. Theoretical aspects of GPCR-ligand complex pharmacology. Chem Rev (2017) 117(1):4–20.
- De Lean A, Stadel J, Lefkowitz R. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J Biol Chem (1980) 255(15):7108–17.
- Bedecarrats GY, Kaiser UB. Mutations in the human gonadotropin-releasing hormone receptor: insights into receptor biology and function. Semin Reprod Med (2007) 25(5):368–78.10.1055/s-2007-984743
- Brioude F, Bouligand J, Trabado S, Francou B, Salenave S, Kamenicky P, et al. Non-syndromic congenital hypogonadotropic hypogonadism: clinical presentation and genotype-phenotype relationships. Eur J Endocrinol (2010) 162(5):835–51.10.1530/EJE-10-0083
- Naor Z. Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor. Front Neuroendocrinol (2009) 30(1):10–29.10.1016/j.yfrne.2008.07.001
- 4. Schally AV, Block NL, Rick FG. Discovery of LHRH and development of LHRH analogs for prostate cancer treatment. Prostate (2017) 77(9):1036–54.10.1002/pros.23360
- Struthers RS, Nicholls AJ, Grundy J, Chen T, Jimenez R, Yen SS, et al. Suppression of gonadotropins and estradiol in premenopausal women by oral administration of the nonpeptide gonadotropin-releasing hormone antagonist elagolix. J Clin Endocrinol Metab (2009) 94(2):545–51.10.1210/jc.2008-1695
- Bliss SP, Navratil AM, Xie J, Roberson MS. GnRH signaling, the gonadotrope and endocrine control of fertility. Front Neuroendocrinol (2010) 31(3):322–40.10.1016/j.yfrne.2010.04.002
- Perrett RM, McArdle CA. Molecular mechanisms of gonadotropin-releasing hormone signaling: integrating cyclic nucleotides into the network. Front Endocrinol (2013) 4:180.
- Tsutsumi R, Mistry D, Webster NJ. Signaling responses to pulsatile gonadotropin-releasing hormone in LbetaT2 gonadotrope cells. J Biol Chem (2010) 285(26):20262–72.
- Choi SG, Jia J, Pfeffer RL, Sealfon SC. G proteins and autocrine signaling differentially regulate gonadotropin subunit expression in pituitary gonadotrope. J Biol Chem (2012) 287(25):21550–60.
- Naor Z, Huhtaniemi I. Interactions of the GnRH receptor with heterotrimeric G proteins. Front Neuroendocrinol (2013) 34(2):88–94.
- White CD, Coetsee M, Morgan K, Flanagan CA, Millar RP, Lu ZL. A crucial role for Galphaq/11, but not Galphai/o or Galphas, in gonadotropin-releasing hormone receptor-mediated cell growth inhibition. Mol Endocrinol (2008) 22(11):2520–30.
- Grosse R, Schmid A, Schoneberg T, Herrlich A, Muhn P, Schultz G, et al. Gonadotropin-releasing hormone receptor initiates multiple signaling pathways by exclusively coupling to G(q/11) proteins. J Biol Chem (2000) 275(13):9193–200.
- Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-releasing hormone receptors. Endocr Rev (2004) 25(2):235–75.
- Munk C, Isberg V, Mordalski S, Harpsoe K, Rataj K, Hauser AS, et al. GPCRdb: the G protein-coupled receptor database – an introduction. Br J Pharmacol (2016) 173(14):2195–207.
- Isberg V, de Graaf C, Bortolato A, Cherezov V, Katritch V, Marshall FH, et al. Generic GPCR residue numbers – aligning topology maps while minding the gaps. Trends Pharmacol Sci (2015) 36(1):22–31.
- Mahoney JP, Sunahara RK. Mechanistic insights into GPCR-G protein interactions. Curr Opin Struct Biol (2016) 41:247–54.10.1016/j.sbi.2016.11.005
- Venkatakrishnan AJ, Deupi X, Lebon G, Heydenreich FM, Flock T, Miljus T, et al. Diverse activation pathways in class A GPCRs converge near the G-protein-coupling region. Nature (2016) 536(7617):484–7.
- Tesmer JJ. Hitchhiking on the heptahelical highway: structure and function of 7TM receptor complexes. Nat Rev Mol Cell Biol (2016) 17(7):439–50.
- Cvicek V, Goddard WA, III, Abrol R. Structure-based sequence alignment of the transmembrane domains of all human GPCRs: phylogenetic, structural and functional implications. PLoS Comput Biol(2016) 12(3):
- Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature (2013) 494(7436):185–94.10.1038/nature11896
- Ballesteros JA, Weinstein W. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci(1995) 25:366–428.10.1016/S1043-9471(05)80049-7
- Huang W, Manglik A, Venkatakrishnan AJ, Laeremans T, Feinberg EN, Sanborn AL, et al. Structural insights into micro-opioid receptor activation. Nature (2015) 524(7565):315–21.
- Flanagan CA, Zhou W, Chi L, Yuen T, Rodic V, Robertson D, et al. The functional microdomain in transmembrane helices 2 and 7 regulates expression, activation, and coupling pathways of the gonadotropin-releasing hormone receptor. J Biol Chem (1999) 274(41):28880–6.
- Katritch V, Fenalti G, Abola EE, Roth BL, Cherezov V, Stevens RC. Allosteric sodium: a key co-factor in class A GPCR signaling. Trends Biochem Sci (2014) 39(5):233–44.
- Zhou W, Flanagan C, Ballesteros JA, Konvicka K, Davidson JS, Weinstein H, et al. A reciprocal mutation supports helix 2 and helix 7 proximity in the gonadotropin-releasing hormone receptor. Mol Pharmacol (1994) 45(2):165–70.
- Trzaskowski B, Latek D, Yuan S, Ghoshdastider U, Debinski A, Filipek S. Action of molecular switches in GPCRs-theoretical and experimental studies. Curr Med Chem (2012) 19(8):1090–109.
- Carpenter B, Nehme R, Warne T, Leslie AG, Tate CG. Structure of the adenosine A(2A) receptor bound to an engineered G protein. Nature (2016) 536(7614):104–7.
- Goncalves JA, South K, Ahuja S, Zaitseva E, Opefi CA, Eilers M, et al. Highly conserved tyrosine stabilizes the active state of rhodopsin. Proc Natl Acad Sci U S A (2010) 107(46):19861–6.
- Ballesteros J, Kitanovic S, Guarnieri F, Davies P, Fromme BJ, Konvicka K, et al. Functional microdomains in G-protein-coupled receptors. The conserved arginine-cage motif in the gonadotropin-releasing hormone receptor. J Biol Chem (1998) 273(17):10445–53.


13 commenti
Every weekend i used to visit this web page, as i want enjoyment,
as this this web site conations actually pleasant funny information too.
It is the best time to make some plans for the future and
it’s time to be happy. I’ve read this post and if
I could I desire to suggest you some interesting things or suggestions.
Maybe you could write next articles referring to this article.
I want to read more things about it!
This paragraph is actually a nice one it
assists new web visitors, who are wishing in favor of blogging.
You ought to take part in a contest for one of the most useful sites on the internet.
I will recommend this blog!
Hello friends, nice post and pleasant urging commented here, I am in fact enjoying
by these.
I enjoy what you guys are up too. This sort of clever work and exposure!
Keep up the amazing works guys I’ve incorporated you guys
to blogroll.
After I initially left a comment I appear to have clicked on the -Notify
me when new comments are added- checkbox and from now on every time a
comment is added I get 4 emails with the exact same comment.
Perhaps there is a way you are able to remove me from that service?
Thank you!
What a information of un-ambiguity and preserveness of valuable experience about unpredicted feelings.
Aw, this was an incredibly nice post. Taking the time and
actual effort to generate a top notch article… but what can I say… I hesitate a lot and never seem to get nearly anything done.
Wow, amazing weblog layout! How long have you been blogging for?
you made running a blog glance easy. The whole look of your website is excellent, as well as the content
material!
Please let me know if you’re looking for a article author for your weblog.
You have some really good articles and I think I would be a good asset.
If you ever want to take some of the load off, I’d love to write some articles for your blog in exchange for a link
back to mine. Please blast me an email if interested.
Thank you!
I have read so many posts about the blogger lovers however this post is in fact a fastidious post, keep it up.
If some onee nseds xpert visw concefning blofging and site-building afterward i proopose him/her tto visit this weeb site, Keeep uup thhe astidious
work.